Probiotics and Gastrointestinal Health
For centuries living microorganisms, particularly lactic acid bacteria (producers of lactic acid from sugar), have been used in food preservation. So there has been a long-term awareness of the beneficial effects of microorganisms. However, the first reports of beneficial effects on human health linked to lactobacilli and bifidobacteria appeared in the late 19th to early 20th century.1
What is a Probiotic?
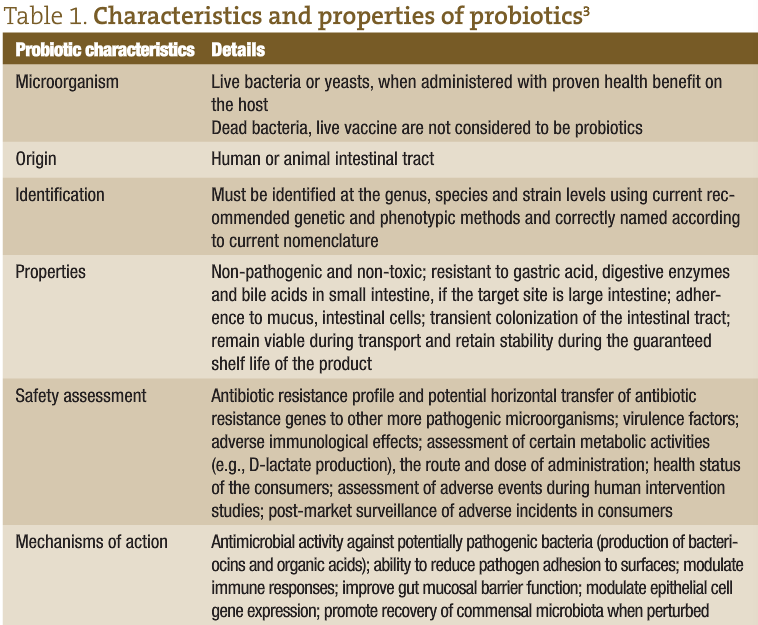
In 1965, Lilly and Stillwell2 proposed the term “probiotics” to refer to ‘substances produced by microorganisms which promote the growth of other microorganisms’ See table 1 for the characteristics and properties of probiotics. The most widely adopted definition of probiotics is ‘live microorganisms which, when administered in adequate amounts, confer health benefits on the host’.4 Health Canada suggests one possibility for probiotics to confer a health benefit would be by modulating the microbiota indigenous to humans.5 Organisms included in probiotic preparations are lactic acid bacteria (such as Lactobacillus acidophilus, L. casei, L. reuteri, L. rhamnosus, Lactococcus lactis), bifidobacteria (eg, Bifidobacterium animalis subsp. lactis, B. bifidum, B. infantis, B. longum), the yeast Saccharomyces boulardii, Enterococcus faecium and Enterococcus faecalis, Escherichia coli Nissle 1917 and Bacillus coagulans (L. sporogenes).
Where Do Probiotics Come From?
The human gastrointestinal (GI) tract has co-evolved with a very complex, stable microbial population (the gut microbiota) of more than 100 trillion microorganisms comprising several hundred different species that has led to the development and optimization of complex immune mechanisms that control this ecosystem. The gut microbiota plays an important role in nutrition and metabolism (digestion, absorption, fermentation, vitamin synthesis and energy production), regulation of immune function (by stimulation of immunoglobulin and cytokine production), and protection (colonization resistance and production of antimicrobial substances). The genomes of the gut microbiota encode for metabolic activities distinct from those of the human microbiota, thus contributing directly to human physiology.
Many factors, such as diet, food poisoning, infections, antibiotic therapy, stress and aging can impact the balance of the gut microbiota, and GI disturbances can range from mild to life-threatening. There is a growing awareness of the importance of the relationship between the gut microbiota and human health and the potential for the manipulation of this relationship to achieve therapeutic effects.
What are the Benefits of Probiotic Use?
The use of the probiotic strains has been widely investigated for a range of conditions, namely, diarrhea in children, antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, traveler’s diarrhea, irritable bowel syndrome (IBS), inflammatory bowel diseases (Crohn’s disease, ulcerative colitis, pouchitis), pancreatitis, necrotizing enterocolitis, colon cancer, allergic diseases including atopic dermatitis (eczema), urogenital infections, Helicobacter pylori gastric infection, prevention of common cold, winter infections, absences from work, lactose intolerance and oral health. The well-characterized immunomodulatory properties of probiotic bacteria have been used as a tool to alleviate intestinal inflammation, normalize gut mucosal dysfunction and down-regulate hypersensitivity reactions.6 In addition, recent work is identifying the role of probiotic organisms in energy metabolism.
Safety of Probiotics
Reports of infection due to lactobacilli and bifidobacteria (the most abundant probiotic strains) are extremely rare and have been estimated to represent only between 0.05%-0.4% of cases of infective endocarditis and bacteraemia, and these mostly in immunocompromised patients. There is no evidence that ingested probiotic lactobacilli or bifidobacteria pose any risk of infection greater than that associated with commensal strains.7 There is a general consensus of opinion that probiotics such as Lactobacilli and Bifidobacteria are suitable and well tolerated. To our knowledge, there are no reports of serious adverse events attributable to probiotics in healthy individuals.
Applications of Probiotics
Probiotic usage is being advocated within all areas of the GI tract and the following is a summary of the range of applications with particular reference to our own studies with antibiotic resistance and probiotics and IBS.
Mouth
The oral cavity is very densely populated with a complex microbiota, where many species have not yet been identified. It is known that 70% of dental plaque (biofilms) contains up to 10 billion bacteria,8 but it is only recently that probiotics have been found to have a potential role in oral health in the prevention and treatment of oral infections, including dental caries (potential to alter colonization of cariogenic bacteria), gingivitis and periodontitis. Krasse et al. found reduction in plaque levels and gingival inflammation with the application of L. reuteri.9 Furthermore, it has been suggested that there is an association between the oral microbiota and systemic disease, such as cardiovascular disease and complications during pregnancy.10 Indirect probiotic actions within the oral cavity include the modulation of aspects of both innate and specific immune function.
Stomach
It is known that about half of the human population carries Helicobacter pylori in the stomach which is acquired in childhood (before the age of 10) and, in the absence of antibiotic therapy, will persist for life.11 H. pylori is associated with an increased risk of developing peptic ulcers, gastric cancer and mucosa-associated lymphoid tissue lymphoma and therapy involves use of a proton-pump inhibitor combined with two antibiotics (clarithromycin, amoxicillin or metronidazole). This therapy is frequently associated with adverse events but the use of probiotics in conjunction with the triple therapy has been found to reduce the frequency of the adverse events; with regard to H. pylori eradication, differences in the effectiveness have been observed depending on the strains used.12,13
Intestine
Within the intestine, the best results relating to the use of probiotics have been obtained for the prevention and treatment of acute diarrhea, including antibiotic-associated and Clostridium difficile-associated diarrhea.12,14 There is also growing evidence for the capacity of probiotics to modulate immune function and reduce intestinal permeability.
Antibiotic Resistance
One of the growing concerns relating to the use of antibiotics is the emergence of antibiotic-resistant microorganisms in response to exposure of the gut microbiota to the antibiotics. On treatment with antibiotics, the balance of the GI microbiota is disturbed and the numbers of organisms present in the gut decreases. However, any antibiotic-resistant strains comprising part of the indigenous microbiota will be able to thrive under such conditions and the regrowth population is often dominated by the antibiotic resistant strains. In a placebo-controlled, double-blind study it has been found that supplementation with probiotics during and post-antibiotic therapy reduces the extent of disruption to the intestinal microbiota with a reduction in the incidence and total numbers of antibiotic-resistant strains in the regrowth population.15,16
Immunity
The intestinal microbiota is considered a positive health asset that exerts a conditional effect on intestinal homeostasis with resident bacteria delivering regulatory signals to the epithelium to instruct mucosal immune responses. The composition of the gut microbiota is believed to influence the development of the immune response. Kelly et al. suggest that an imbalance between aggressive and protective bacterial species, or loss of gut bacteria that promote tolerance and Treg cell polarization could lead to excessive Th1 or Th2 responses, thus promoting inflammatory or allergic diseases.17
The recognition of the involvement of the gut microbiota (and more specifically lactobacilli and bifidobacteria) in the development of allergic diseases has led many researchers to develop strategies to modify gut microbiota. Several studies have shown a benefit in the prevention of atopic dermatitis in infants and older children, with a clear effect only for the prevention of eczema.18,19 Benefits of probiotic supplementation on the symptoms of allergic rhinitis have also been observed,20 but not for asthma treatment. However, the modulation of immune response and inflammatory markers following probiotic administration has been widely recorded.
IBS
Within the intestine it has been found that probiotic supplementation shows promise with IBS, a complex, multifactorial GI disorder that is a female dominated condition with female:male ratio of 2-2.5:1.21
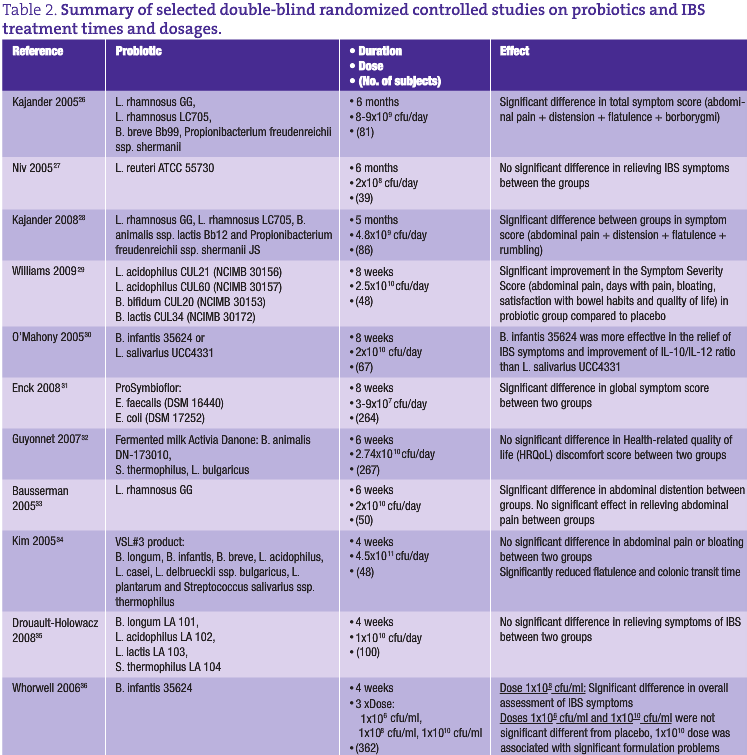
The prevalence of IBS in the general population varies from 3% to 25%. In the United States, IBS affects 15 million adults, in Canada more than 2 million people, and in the United Kingdom 10-15% of the adult population is affected.22,23 IBS pathogenesis is far from clearly defined and most hypotheses focus on one or more of the following: altered intraluminal milieu, immune activation, enteric neuromuscular dysfunction and/or brain-gut axis dysregulation. The pharmacological therapy for IBS can involve antidiarrheals, laxatives, antispasmodics, antidepressant and serotonergic agents,24 but success with these drugs has been limited (some are associated with safety issues) and, hence, it is not surprising that many IBS sufferers are turning to complementary and alternative therapies, such as probiotics, peppermint oil, soluble fiber, hypnotherapy and cognitive-behavioral therapy.25 An overview of studies performed with probiotics in IBS treatment is shown in Table 2.
IBS may result from a dysfunctional interaction between the indigenous flora and the intestinal mucosa leading to immune activation in the colonic mucosa and changes in the colonic microbiota could result in the proliferation of gas-producing organisms, or in organisms that facilitate deconjugation of bile acids impacting upon water and electrolyte transport within the colon. Quigley et al. reviewed the use and efficacy of probiotics in IBS and suggested a clear rationale for probiotic usage in response to a dysfunctional relationship between the indigenous microbiota and the host.37 The authors further suggested the feasibility of probiotics for bacterial displacement and alteration of luminal content. However, clarification is required regarding the need for clear definition of strains, dosage and viability of the probiotic organisms in use. Kassinen et al. have shown lower numbers of both lactobacillus spp. and bifidobacteria in the microbiota of IBS sufferers suggesting the need for a product comprising both organisms.38
The use of a probiotic comprising both Lactobacillus acidophilus and Bifidobacterium spp. (LAB4) in a double-blind, placebo-controlled trial resulted in a significantly greater improvement in the Symptom Severity Score of IBS and in scores for Quality of Life, Days with Pain and Satisfaction with Bowel Habit over the 8-week intervention period in the volunteers receiving the probiotic.29
Conclusion
The scope of probiotics seems endless. It seems that more and more applications for the involvement of probiotic supplements are being identified, almost on a daily basis. There are inconsistencies in the results that have been obtained, but much of that relates to the testing of a diversity of products with different compositions and potencies. It is apparent that there are benefits from probiotics across a broad spectrum of conditions, many of which are linked with antibiotic therapy. As our knowledge of the value of the GI microbiota is growing, so is the awareness of the potential for probiotics, and as the mechanistic details of the mode of action of probiotics becomes clearer, further developments will occur. Overall, the message for probiotics and the GI tract is “watch this space”.
Sue Plummer BSc, PhD is a Research Director at Cultech Limited, one of the most successful entrepreneurs in Wales, UK. She and her husband Nigel set up the company in 1994 and, as a result of its scientific expertise; it has since grown rapidly to become one of the UKs leading manufacturers of food supplements and probiotic products.Her main research interest is in the effects of probiotics, fatty acids and plant antimicrobials on G.I. tract physiology and disease. She has written and co-written numerous articles and papers.She is a graduate in Microbiology from London, UK and has her doctorate in Microbial Ecology from Cardiff, UK.
 Iveta Garaiova MSc, PhD is a Research Manager at Obsidian Research Ltd, UK involved in research of gut microbiota, antibiotic resistance and role of probiotics in prevention and treatment of gastrointestinal diseases. Dr Garaiova has co-written various papers and presented at international scientific symposia. She has her doctorate in Medical Biochemistry from Comenius University, Bratislava, Slovakia.
Iveta Garaiova MSc, PhD is a Research Manager at Obsidian Research Ltd, UK involved in research of gut microbiota, antibiotic resistance and role of probiotics in prevention and treatment of gastrointestinal diseases. Dr Garaiova has co-written various papers and presented at international scientific symposia. She has her doctorate in Medical Biochemistry from Comenius University, Bratislava, Slovakia.
 Marija Pevac-Djukic, MD, ND was born and raised in Serbia where she earned her M.D. degree from The University of Belgrade in 1989. After practicing for 4 years as a G.P. she immigrated to Canada. When Marija discovered naturopathic medicine she was happy to learn that this philosophy resonated with her medical background and her belief system. After graduating from The Canadian College of Naturopathic Medicine, Marija joined Seroyal, where she works as a Medical Advisor part-time. She also has a private practice in Toronto.
Marija Pevac-Djukic, MD, ND was born and raised in Serbia where she earned her M.D. degree from The University of Belgrade in 1989. After practicing for 4 years as a G.P. she immigrated to Canada. When Marija discovered naturopathic medicine she was happy to learn that this philosophy resonated with her medical background and her belief system. After graduating from The Canadian College of Naturopathic Medicine, Marija joined Seroyal, where she works as a Medical Advisor part-time. She also has a private practice in Toronto.
References
1. Tannock GW. Probiotics: time for a dose of realism. Curr Issues Intest Microbiol. 2003;4(2):33-42.
2. Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747-748.
3. Sanders ME. How do we know when something called “Probiotic” is really a probiotic? A guideline for consumers and health care professionals. Functional Food Rev. 2009;1(1):3-12.
4. Joint FAO/WHO Expert Consultation. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Food and Agriculture Organization of the United Nations and World Health Organization, October 2001. http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf. Accessed November 21, 2009.
5. Health Canada. Natural Health Product Monograph – Probiotics 2009. Health Canada Web site. http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/monograph/mono_probioti-eng.php. Accessed November 21, 2009.
6. Collado MC, et al. The impact of probiotic on gut health. Curr Drug Metab. 2009;10(1):68-78.
7. Borriello SP, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis. 2003;36(6):775-780.
8. Teughels W. Oral flora and oral care with probiotics. The 5th International Yakult Symposium, Amsterdam, NL, 2009.
9. Krasse P, et al. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J. 2006;30(2):55-60.
10. Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009;33(1):8-13.
11. Cover TL, Blaser MJ. Helicobacterpylori in health and disease. Gastroenterology. 2009;136(6):1863-1873.
12. Lenoir-Wijnkoop I, et al. Probiotic and prebiotic influence beyond the intestinal tract. Nutr Rev. 2007;65(11):469-489.
13. Sullivan A, Nord CE. Probiotics and gastrointestinal diseases. J Intern Med. 2005;257(1): 78-92.
14. Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des. 2009;15(13):1428-1518.
15. Plummer SF, et al. Effects of probiotics on the composition of the intestinal microbiota following antibiotic therapy. Int J Antimicrob Agents. 2005;26(1):69-74.
16. Madden JAJ, et al. Effect of probiotics on preventing disruption of the intestinal microflora following antibiotic therapy: A double blind, placebo controlled pilot study. Int Immunopharmacol. 2005,5(6):1091-1097.
17. Kelly D, et al. Commensal gut bacteria: mechanisms of immune modulation. Trends Immunol. 2005;26(6):326-333.
18. Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007;4:CD006475 Review.
19. Reid G. Probiotics and prebiotics—progress and challenges. Int Dairy J. 2008;18(10-11):969-975.
20. Vliagoftis H, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101(6):570-579.
21. Adeyemo MA, Chang L. New treatments for irritable bowel syndrome in women. Womens Health (Lond Engl) 2008;4(6):605-623.
22. McFarland LV. State-of-the-art of irritable bowel syndrome and inflammatory bowel disease research in 2008. World J Gastroenterol. 2008;14(17):2625-2629.
23. Clement T (Minister of Health, Canada). Irritable Bowel Syndrome Awareness Month – April, 2008. Health Canada Website. http://www.hc-sc.gc.ca/ahc-asc/minist/messages/2008_04_24-eng.php. Accessed November 11, 2009.
24. Hammerle CW, Surawicz CM. Upades on treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14(17):2639-2649.
25. Shen YH, Nahas R. Complementary and alternative medicine for treatment of irritable bowel syndrome. Can Fam Physician. 2009;55(2):143-148.
26. Kajander K, et al. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22(5):387-394.
27. Niv E, et al. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome – a double blind, placebo-controlled, randomized study. Clin Nutr. 2005;24(6):925-931.
28. Kajander K, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27(1):48-57.
29. Williams E, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97-103.
30. O’Mahony L, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005;128(3):541-551.
31. Enck P, et al. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome – a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008;20(10):1103-1109.
32. Guyonnet D, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26(3):475-486.
33. Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147(2):197-201.
34. Kim HJ, et al. A randomized controlled trial of a probiotic combination VSL#3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil 2005;17(5):687-696.
35. Drouault-Holowacz S, et al. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol 2008;32(2):147-152.
36. Whorwell PJ, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581-1590.
37. Quigley EM, Flourie B. Probiotics and irritable bowel syndrome: a rationale for their use and an assessment of the evidence to date. Neurogastroenterol Motil 2007;19(3):166-72.
38. Kassinen A, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33.










