Chris D. Meletis, N.D.
A clinical framework for restoring adrenal and gonadal hormone balance by supporting mitochondrial bioenergetics, antioxidant capacity, and adaptogenic stress resilience—upstream of testosterone, cortisol, and thyroid therapies.
Abstract
This article explores how mitochondrial function underpins adrenal and gonadal steroidogenesis, contributing to hypogonadism, low libido, and adrenal insufficiency when impaired. It outlines assessment strategies and integrative therapies—including mitochondrial nutrients, antioxidants, adaptogens, lifestyle, and thyroid support—to build a resilient foundation for long-term hormone optimization
There’s an age old question, is it better to give a man a fish or teach him to fish? Well, the answer is complex when it comes to hormone optimization. As naturopathic physicians and functional medicine providers, we must assess the individual physiological complexity, compliance, and commitment of the patient to address the underlying etiologies that have led to suboptimal adrenal or gonadal function. The application of the principal philosophy of treating the cause, Tolle causam, is the ideal answer if the patient can address full body wellness.
Frequently, the cause is an underrecognized factor such as mitochondria dysfunction. Effective management of hypogonadism, low libido, or adrenal insufficiency requires careful consideration of both mitochondrial energetics and adrenal function, as these two systems form the biochemical foundation of steroidogenesis.1 Data indicate mitochondrial dysfunction and adrenal dysregulation as primary etiological factors underlying declines in sex hormone synthesis.1,2
Supporting mitochondrial function is paramount to addressing a patient’s physiological resilience and capacity to handle anabolic and catabolic adrenal influences. As both hormonal pathways impact distal cells that must also have the mitochondrial capacity in the form of ATP energy to optimally respond to upregulation. Effective mitochondrial capacity also allows for a more abundant hormonal presence, which affects the 37 trillion cells that comprise the patient sitting in front of us. Although this article is presented along with this month’s male-health-themed offerings, the same applies for female hormonal balance and health as well.
Mitochondrial Site of Steroidogenesis
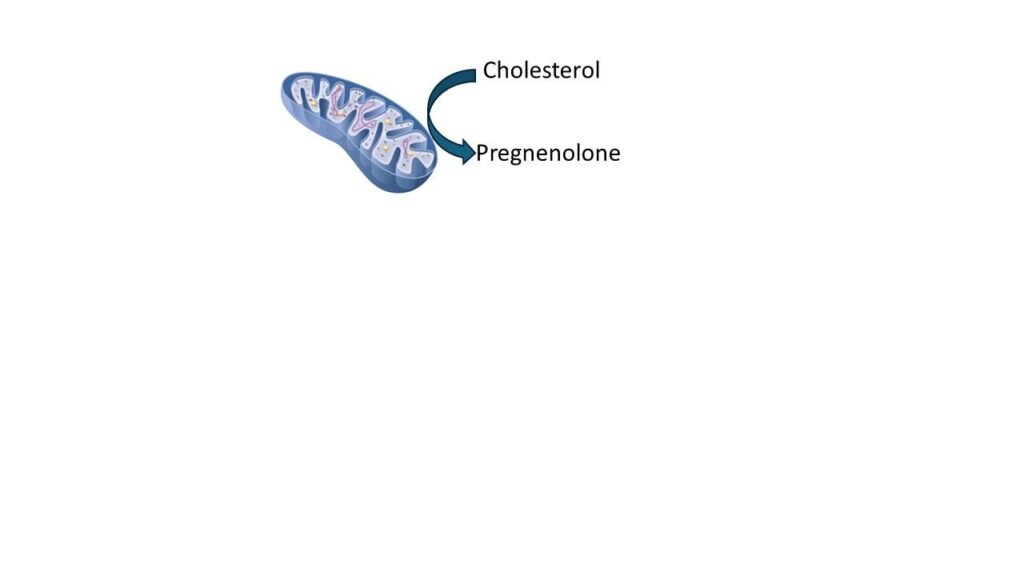
Steroid hormones arise within mitochondria through the conversion of cholesterol to pregnenolone by cytochrome P450 side-chain cleavage enzyme (CYP11A1) located on the inner mitochondrial membrane.3 Impaired electron transport or reduced mitochondrial membrane potential directly limits steroid output.4 Proper function of the StAR protein is also necessary for cholesterol import, and its deficiency results in combined adrenal and gonadal failure.5
Histologic studies show that adrenal mitochondria are also linked to adrenal medulla function. All too often we fall into the trend of treating and supporting the adrenal cortex, yet the adrenal medulla is also by intention part of adrenal responsiveness and healthy physiology.6 CRF (corticotropin-releasing factor) increases the spontaneous discharge rate of locus coeruleus (LC) neurons and enhances norepinephrine (NE) release in the prefrontal cortex whereas glucocorticoids seem to exert more inhibitory effects on NE release.7,8 Those same neurotransmitters are released from the adrenal medulla in times of stress. This is one of the reasons that patients experience the “wired but tired” syndrome with lower than healthy physiological adrenal glucocorticoid production.
Diseases producing variants of CYP11A1 or membrane fusion proteins such as OPA1 often manifest as both adrenal insufficiency and hypogonadism.6
Sex Hormone Regulation of Mitochondrial Biogenesis
Sex steroids reciprocally modulate mitochondrial biogenesis. Androgens activate peroxisome proliferator-activated receptor gamma co-activator (PGC-1α) transcription factor, stimulating mitochondrial DNA (mtDNA) replication.9,10 Studies demonstrate restoration of brain mitochondrial function following testosterone therapy in aging men.11 Estrogens exert similar protective control on the mitochondria.12 I often remind my older male patients with age-matched female partners that it is not uncommon to see a serum estradiol level for males being equal or higher than their female counterpart if she is not on hormone replacement.
Adrenal Cortex as a Mitochondria-Dense Organ
The adrenal cortex contains one of the highest mitochondrial densities of any endocrine tissue due to its steroidogenic demands.4,13 Adrenocortical mitochondria integrate bioenergetics and steroid metabolism, and disruption of membrane potential or phospholipid remodeling has been linked to glucocorticoid deficiency.13 Chronic stress triggers sustained activation of the hypothalamic–pituitary–adrenal axis, which preferentially directs cholesterol toward cortisol synthesis.14 This persistent diversion, known as pregnenolone steal, underscores the vulnerability of steroidogenesis to mitochondrial exhaustion.
Mitochondrial Dysfunction in Endocrine Pathophysiology
Mitochondrial disorders commonly present with multisystem endocrine manifestations—adrenal insufficiency, hypogonadism, and thyroid secondary suppression—illustrate the universal requirement of mitochondrial integrity for steroid biosynthesis.15 I make it a point to educate my patients that hormones by their nature have broad and far-reaching effects distally from the gland of origin. When supported with botanical, diet, lifestyle, nutraceuticals, or pharmaceuticals, the targeted tissues must also have sufficient mitochondrial function to take up and utilize the nutrients being therapeutically offered. After all, it takes energy to regain and sustain homeostasis.
Clinical Assessment
Evaluation of adrenal-mitochondrial integrity includes measuring serum pregnenolone and DHEA-S as indicators of steroidogenic reserve. Lactate-to-pyruvate ratios and urinary organic acids help infer mitochondrial redox efficiency, while the DHEA/7-keto-DHEA ratio reflects HPA oxidative activity. Persistent low ratios can suggest mitochondrial over-oxidation and cortisol dominance. I routinely perform serum pregnenolone, DHEA, and DHEA sulfate labs for patients to assess their upstream capacity and reserve, and to look for potential “individual” hurdles that lead them to needing clinical intervention. I also routinely include both urinary and salivary hormone testing as well.
Therapeutic Framework
Restoration of mitochondrial capacity provides the biochemical foundation for endocrine recovery. Evidence supports the following sequence of interventions:
- Bioenergetic repletion with coenzyme Q10 (CoQ10), acetyl-l-carnitine, riboflavin, nicotinamide riboside, α-lipoic acid, and creatine to restore oxidative phosphorylation.16
- Mitochondrial antioxidant therapy using N-acetylcysteine, glycine, mitoquinol, or glutathione to prevent ROS-induced enzyme inhibition.17,18
- Adrenal adaptogenic modulation through Rhodiola rosea and Withania somnifera can normalize cortisol rhythm.19-21 Often as clinicians we have our tried and proven combinations of adaptogenic botanicals that we have incorporated into our clinical protocols. I routinely impart the philosophical tenet that “a penny saved is a penny earned.” Or put another way, that conservation of adrenal output with the use of L-theanine can help a patient stay cool, calm, and collected in order to reduce the impact of stress on the mitochondria and hormones.
Another means of adaptogenic modulation is to use proprietary blends such as combination of Magnolia officinalis and Phellodendron amurense that was shown in a 2013 article to help with cortisol imbalance.22 After four weeks of supplementation, salivary cortisol exposure was significantly (p<0.05) lower (−18%) in the Relora group compared to placebo. Compared to placebo, the Relora group had significantly better (p<0.05) mood state parameters, including lower indices of Overall Stress (−11%), Tension (−13%), Depression (−20%), Anger (−42%), Fatigue (−31%), and Confusion (−27%), and higher indices of Global Mood State (+11%) and Vigor (+18%).
- Embracing the concept of treating upstream by using hormone precursor replacement with physiological pregnenolone or DHEA with refinement of dosages using pre and post lab monitoring. After all, both pregnenolone and DHEA confer important independent beneficial physiological effects in the body independent of downstream hormones of testosterone, estrogen, or progesterone, which is the all-too-often primary goal to be addressed in patients. Yet, our philosophical approach is treating the entire body and not just seeking the quick fix that was suggested by a patient’s Google or AI search or podcast they recently watched. There is a potential time for cookbook medicine, yet it is our years of training and holistic approach that gives our patients that extra edge to thrive in contrast to simply surviving. We are in our patients’ lives for the long game.
- Lifestyle and circadian restoration, as sleep deprivation and glycemic instability worsen mitochondrial health.23,24 Testing for sleep apnea, addressing snoring of bed partners that can keep patients awake, controlling blue light exposure, etc. are all paramount.
The Thyroid-Adrenal-Mitochondrial Interdependence
Thyroid hormone (T3) acts as a master regulator of mitochondrial biogenesis.25 Hypothyroidism thus compounds mitochondrial insufficiency and produces biochemical patterns resembling adrenal fatigue.26 It is also noteworthy that T3 hormone and T2 hormone have a stimulatory effect on mitochondrial function:27 Yet another reason to consider porcine-based thyroid replacement therapy. T2 hormone is not a standardized approach, but it is offered along with the standardized T3 and T4 in commercially available thyroid replacement pharmaceutical offerings.28,29
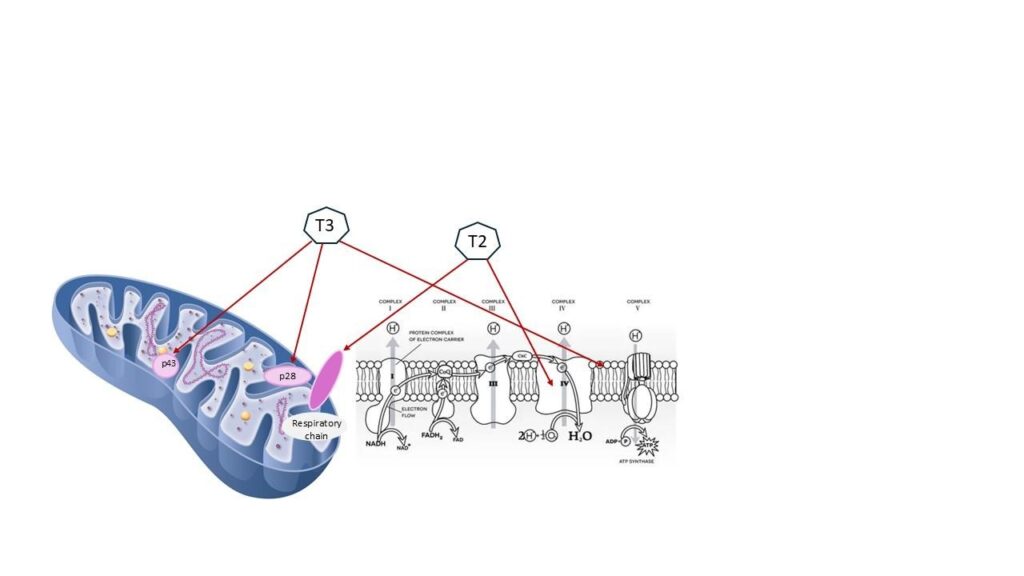
We must remind our patients that when we address hypothyroidism and bring thyroid laboratory indices within the normal range, we have literally increased their basal metabolic rate, which routinely alters metabolism of hormones, medications clearance, digestion, and energetic demand including adrenal reserve.
Conclusion
There is an interplay between the mitochondria, steroid production, and adrenal health. Supporting the health of the mitochondria is essential for healthy testosterone levels and adrenal function. The mitochondria’s role in production of testosterone and other hormones is a little-considered aspect of men’s health, but one that can make a significant difference in treatment outcomes.

Chris D. Meletis, ND is an educator, international author, and lecturer. He has authored 18 books and more than 200 scientific articles in prominent journals and magazines. Dr Meletis served as Dean of Naturopathic Medicine and CMO for 7 years at NUNM. He was recently awarded the NUNM Hall of Fame award by OANP, as well as the 2003 Physician of the Year by the AANP. Dr Meletis spearheaded the creation of 16 free natural medicine healthcare clinics in Portland, OR. Dr Meletis serves as an educational consultant for Fairhaven Health, Berkeley Life, TruGen3, US BioTek, and TruNiagen. He has practiced in Beaverton, OR, since 1992.
References:
1. Papadopoulos V, Miller WL. Role of mitochondria in steroidogenesis. Best Pract Res Clin Endocrinol Metab. 2012;26(6):771-790.
2. Melchinger P, Garcia BM. Mitochondria are midfield players in steroid synthesis. Int J Biochem Cell Biol. 2023;160:106431.
3. Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379(1-2):62-73.
4. Vega-Vásquez T, Langgartner D, Wang JY, Reber SO, Picard M, Basualto-Alarcón C. Mitochondrial morphology in the mouse adrenal cortex: Influence of chronic psychosocial stress. Psychoneuroendocrinology. 2024;160:106683.
5. Horikawa R. [Congenital lipoid adrenal hyperplasia]. Nihon Rinsho. 2004;62(2):351-356.
6. Neuhaus JF, Baris OR, Kittelmann A, Becker K, Rothschild MA, Wiesner RJ. Catecholamine Metabolism Induces Mitochondrial DNA Deletions and Leads to Severe Adrenal Degeneration during Aging. Neuroendocrinology. 2017;104(1):72-84.
7. Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993;697:173-188.
8. Smagin GN, Swiergiel AH, Dunn AJ. Corticotropin-releasing factor administered into the locus coeruleus, but not the parabrachial nucleus, stimulates norepinephrine release in the prefrontal cortex. Brain Res Bull. 1995;36(1):71-76.
9. Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79(2):208-217.
10. Gharahdaghi N, Rudrappa S, Brook MS, et al. Testosterone therapy induces molecular programming augmenting physiological adaptations to resistance exercise in older men. J Cachexia Sarcopenia Muscle. 2019;10(6):1276-1294.
11. Yan W, Zhang T, Kang Y, et al. Testosterone ameliorates age-related brain mitochondrial dysfunction. Aging (Albany NY). 2021;13(12):16229-16247.
12. Malik S, Chakraborty D, Agnihotri P, et al. Mitochondrial proteomics reveals the impact of Estrogen in enhancing energy metabolism of patient-derived fibroblast-like synoviocytes in rheumatoid arthritis. Inflamm Res. 2025;74(1):146.
13. Jefcoate C. High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest. 2002;110(7):881-890.
14. Lauren Thau JG, Sandeep Sharma. Physiology, Cortisol. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK538239/. Published 2023. Accessed October 24, 2025.
15. Duarte A, Poderoso C, Cooke M, et al. Mitochondrial fusion is essential for steroid biosynthesis. PLoS One. 2012;7(9):e45829.
16. Dietary Supplements for Primary Mitochondrial Disorders: Fact Sheet for Health Professionals. National Institutes of Health. https://ods.od.nih.gov/factsheets/PrimaryMitochondrialDisorders-HealthProfessional/. Published 2020. Accessed October 24, 2025.
17. Kumar P, Liu C, Suliburk J, et al. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J Gerontol A Biol Sci Med Sci. 2023;78(1):75-89.
18. Abdeahad H, Moreno DG, Bloom SI, Norman L, Lesniewski LA, Donato AJ. MitoQ reduces senescence burden in doxorubicin-treated endothelial cells by reducing mitochondrial ROS and DNA damage. Am J Physiol Heart Circ Physiol. 2025;329(5):H1154-h1161.
19. Panossian A, Wikman G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol. 2009;4(3):198-219.
20. Olsson EM, von Schéele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75(2):105-112.
21. Lopresti AL, Smith SJ, Malvi H, Kodgule R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine (Baltimore). 2019;98(37):e17186.
22. Talbott SM, Talbott JA, Pugh M. Effect of Magnolia officinalis and Phellodendron amurense (Relora®) on cortisol and psychological mood state in moderately stressed subjects. J Int Soc Sports Nutr. 2013;10(1):37.
23. Dassanayaka S, Readnower RD, Salabei JK, et al. High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem J. 2015;467(1):115-126.
24. Luo Z, Ji R, Ye R, Shi Y, Pang Q, Yin M. Reduced serum levels of mitochondria-derived peptide MOTS-c in patients with obstructive sleep apnea. Sleep Biol Rhythms. 2025;23(3):305-311.
25. Anthonsen S, Larsen J, Pedersen PL, Dalgaard LT, Kvetny J. Basal and T₃-induced ROS production in lymphocyte mitochondria is increased in type 2 diabetic patients. Horm Metab Res. 2013;45(4):261-266.
26. Romão JS, Neto JGO, Andrade CBV, Carvalho JJ, Pazos-Moura CC, Oliveira KJ. Hypothyroidism modulates mitochondrial dynamics and mitophagy in the heart of rats under fed and fasting conditions. Life Sci. 2024;359:123254.
27. Goglia F, Moreno M, Lanni A. Action of thyroid hormones at the cellular level: the mitochondrial target. FEBS Lett. 1999;452(3):115-120.
28. Zucchi R, Rutigliano G, Saponaro F. Novel thyroid hormones. Endocrine. 2019;66(1):95-104.
29. Maria Moreno M, Giacco A, Di Munno Celia Goglia F. Molecular and Cellular Endocrinology. 2017;458(15):121-126