Jillian Stansbury, ND
Spending half the year in third-world countries for the past 5 years has put traveler’s diarrhea high on my radar. Statistics have shown that traveler’s diarrhea is almost part of the price of traveling to exotic locales and is somewhat hard to avoid when venturing outside of cloistered 5-star hotels. And if you are not going to do that, why go there at all? The longer you stay and the more food you eat, the more likely it becomes that you will pick something up. This article focuses on the research and practices in beating those odds.
Bacteria are responsible for at least 50% of all acute cases of traveler’s diarrhea, with viruses being the second most likely causative organism and protozoa being to blame in less than 10% of cases.1 Of the causative bacteria, Escherichia coli strains are the most common culprit, but Salmonella, Campylobacter, and Shigella are also possible causes of traveler’s diarrhea. Enterotoxigenic E coli can be isolated from about 50% of people afflicted.2 Enterotoxigenic Bacteroides fragilis is another less well-known potential bacterial pathogen recently identified as being associated with traveler’s diarrhea. Arcobacter is another bacterial pathogen similar to Campylobacter.3 In some locales, intestinal parasites and viruses, including arbovirus and Norwalk viruses, may be etiologic factors.4 Norovirus is another viral pathogen that can cause acute diarrhea, is associated with 7% of diarrhea-related deaths in the United States, and has been identified in more than 15% of Europeans returning from travel abroad.5 Microsporidiosis may also be the culprit. Microspores are obligate intracellular microorganisms and were previously classified as protozoans but recently were reclassified as fungi. Enterocytozoon bieneusi and Encephalitozoon intestinalis are 2 microspores known to infect humans and cause diarrhea and systemic disease.6 Although these bacteria can be considered causative pathogens, traveler’s diarrhea may be multifactorial and a reaction to many microbial strains foreign to the intestinal ecosystem at once. The bacteria aforementioned are harmful to the intestinal cells, damaging and inflaming them, but other factors described herein may weaken or strengthen the ability of intestinal mucosal cells to resist the number of pathogenic bacteria and shifts in the overall ecosystem.
Pathogenesis of Traveler’s Diarrhea
Symptoms of acute infectious diarrhea can be mild such as loose stools only, without systemic symptoms, or may be more severe with fever and malaise, explosive diarrhea, nausea, and vomiting. When severe and of significant duration, vomiting and diarrhea can lead to electrolyte imbalance, vascular collapse, and renal failure. Sodium depletion may lead to metabolic acidosis, and potassium depletion may lead to cardiac excitability. Excessive mucous secretions may contain large amounts of potassium. Magnesium depletion is also possible and is associated with nerve excitability and risk of tetany and cardiac abnormalities. Infants, older persons, and debilitated individuals are especially susceptible to acute dehydration and to resultant electrolyte imbalance complications. Some persons may be extremely susceptible to diarrheal infections, while others are somewhat resistant. For unknown reasons, women are more susceptible to traveler’s diarrhea than men.7 The susceptibility may have less to do with systemic immune function and more to do with intestinal health and a well-established intestinal flora ecosystem. The strength of the intestinal mucosa may discourage bacteria from adhering. Escherichia coli and other gut pathogens attach to enterocytes, collapse the microvilli of infected cells, and alter numerous absorptive and other cellular functions. Secretory diarrhea results as these injured cells release numerous small molecules through the gap junctions between the intestinal mucosal cells.8 The strength of the gap junctions between mucosal cells may affect susceptibility to E coli and other enteric pathogens. Variations in lactoferrin, osterprotegerin, and interleukin 10 genes may slightly increase the risk of traveler’s diarrhea.7 Specific genes have also been identified that control the biological rhythms of intestinal motility and promote more activity on awakening in the morning and less activity while asleep at night. Some researchers have proposed that travelers are more susceptible to diarrhea because of disruption of the biological clock, including intestinal motility rhythms.9
Hospitalization, intravenous fluids, and antibiotics are occasionally necessary to treat traveler’s diarrhea. In most cases, however, treatment is often palliative and supportive as the body adjusts to the sudden alteration in gut flora, and most individuals will recover in a few days.
Prevention of Traveler’s Diarrhea
The self-limiting nature of this illness is a less than satisfactory outcome that leaves us wanting something better for prevention, particularly if you travel often or do not want to spend several days of your short vacation with less than optimal health. There have been reports of a transdermal vaccine against E coli that is able to reduce the severity of traveler’s diarrhea but does not prevent it.10
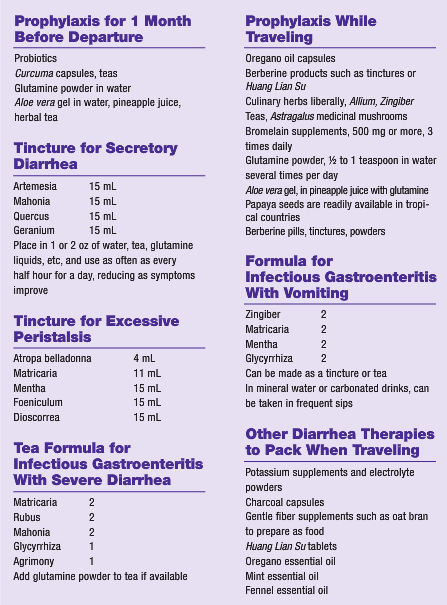
Glutamine may help reduce endotoxin-induced mucosal injury.11 Agents such as glutamine and curcumin from Curcuma longa (turmeric) may help tighten gap junctions between intestinal epithelial cells and improve intestinal health prophylactically.12 Glutamine occurs naturally in significant amounts in Aloe vera and in small amounts (one-tenth of what is in A vera) in Ananas comosus (pineapple).13 Aloe vera leaf products and pineapple juices, especially if fresh, may be consumed as preventives.
Experienced travelers have long said “Don’t drink the water.” Brush your teeth with bottled water, and keep your lips zipped in the shower. If you are really off the grid, you must haul a water filter, and add water preparation to your daily activities. Also, do not eat raw foods or salads. The problem is not those foods but rather the fact that lettuce, tomatoes, or other raw salads and dishes are prepared after washing the produce in tap water. The cups and plates themselves may also be an issue. Dishes are not typically washed in bottled or scrupulously boiled tap water. Even ½ teaspoon of water in the bottom of a bowl or glass could be enough to upset the intestinal ecosystem. Furthermore, while boiling water will kill bacteria, it may simply cause some parasites to morph into cystic forms. Some researchers have reported that well-performed “food hygiene” (scrupulous attention to boiling and washing techniques) is not entirely effective in preventing infectious diarrhea.14 No matter how careful we are, a large percentage of travelers experience some degree of digestive upset.
Preparing the intestines ahead of time with probiotics seems logical, and the extensive literature on the symbiotic relationships between our intestinal flora and individual microbes, cellular junctions, enzymes, systems, immune, and inflammatory activity, and so forth will not be detailed herein. Suffice it to say that the research is vast and complex and that healthy intestinal ecosystems are fundamental to intestinal health, as well as to that of the entire body, as all systemic conditions (arthritis and joint pain, skin disease, autoimmune disease, etc) are associated with leaky gut.
The proteolytic enzyme bromelain from pineapples has been shown to affect enterocyte receptors for infectious strains of E coli in a manner that inhibits the ability of the bacteria to bind intestinal cells.15 Thus, fresh pineapple juices and extracts may be helpful in addition to the glutamine they contain. Glutamine had been shown to help heal leaky gut16 and may improve ulcerative lesions in the intestines. Because glutamine powder is inexpensive and easy to travel with, it is useful as a preventive measure. One-half to 1 teaspoon stirred into water or herbal tea may be taken 3 times daily starting as early as 1 month before international departure. A folkloric remedy for intestinal infections and parasites has been to dry papaya seeds and grind them into a powder to use as a condiment in foods. Carica papaya (papaya) seed extracts have been shown to have activity against E coli, Staphylococcus aureus, Salmonella typhi, and Pseudomonas aeruginosa.17 Inclusion of liberal amounts of the antimicrobial culinary spices Allium (garlic), Zingiber (ginger), and C longa in the diet is also encouraged.
Berberine Alkaloids, Essential Oils, and Herbal Therapies for Infectious Diarrhea
For acute diarrheal symptoms, it is important to keep fluid intake high and to replace the electrolytes being lost but to avoid acidic fruit juices, milk, or any other fluid that could potentially irritate the inflamed mucosal membrane cells. Small sips of carbonated liquids or water with glutamine powder may be best tolerated. Foods such as oat bran or cooked rice that absorb excessive liquid may be useful. Psyllium powders or other fiber powders stirred into liquids may be helpful to some but can be irritating to those with preexisting irritable bowel.
The berberine alkaloids are a group of isoquinoline alkaloids that are among our best herbal therapies for infectious diarrhea. The berberine family of alkaloids includes berberine in Mahonia, hydrastine in Hydrastis, phellodendrine in Phellodendron, and coptine in Coptis, and all have an affinity for secretory infections of mucous membranes. Several investigations have identified extensive antimicrobial properties for these herbs.18 Coptis (also known as Coptidis) has been used in China to treat secretory diarrhea and gastroenteritis for more than 1000 years. Coptis roots contain at least 12 protoberberine alkaloids, recently reported to be transformed by phase 1 and phase 2 metabolism and ultimately excreted via the urine.19 Huang Lian Su is a phellodendrine-containing pill available from vendors of Traditional Chinese Medicine, and it is easy to travel with small vials of the tiny pills, even for backpackers. Berberis species have also shown activity against the amoeba Entamoeba histolytica, as have Tinospora, Terminalia, and Zingiber.20
Several miscellaneous herbs have shown promise as infectious diarrhea treatment in modern research arenas. Ergosterol is a common, if not ubiquitous, constituent of medicinal mushrooms. Ergosterol has been shown to reduce degranulation of mast cells, a mechanism that may contribute to the broad anti-inflammatory effects of Poria, Polyporus, Reishi, Cordyceps, and many medicinal fungi.21 Mast cell secretions may help initiate the opening of the gap junctions between mucosal cells; therefore, decreasing their activation may reduce secretory diarrhea and damage to mucosal cells. Capsules of these mushrooms or teas may be preventive and therapeutic.
Essential oils may also be among some of our powerful tools for acute infections, and essential oil of Origanum vulgare (oregano) has been shown to be one of the strongest and broadest-acting of the common and inexpensive essential oils. Anti-infective activity against Bacillus subtilis, Enterobacter cloacae, E coli, Micrococcus flavus, Proteus mirabilis, P aeruginosa, Salmonella enteritidis, Staphylococcus epidermidis, Salmonella typhimurium, and S aureus has been demonstrated.22 While many chemical constituents in common Lamiaceae (mint) family essential oils are active against these microbes, carvacrol has shown the highest antibacterial activity.23,24 Apium graveolans (celery), Foeniculum (fennel), and other umbels may alleviate gas, cramping, and nausea and may have antimicrobial effects of their own.25 Grinding these seeds and using them in the diet, carrying tinctures, and/or consuming essential oils of these plants may be preventive and therapeutic. These essential oils and mint and oregano oils may also be comforting for gas, bloating, and intestinal cramping when applied topically and covered with heat or when placed in castor oil and rubbed into the abdomen. Essential oils make great travel medicines, as they pack a punch with a minimum of weight and size, can be used topically and internally, and have long shelf lives when well protected.
Treatment of Traveler’s Diarrhea
Technically, diarrhea is classified into several types, and intestinal infections typically promote a secretory type of diarrhea. As already explained, bacterial endotoxins commonly promote excessive secretion of fluids and electrolytes associated with absorption difficulties and damage to the mucosal cells, causing abundant watery fluid in the intestinal lumen. Hence, therapy for secretory diarrhea may involve symptomatic treatment such as astringent herbs. When it is necessary to check excessive fluid losses, astringents such as Rubus leaves, Geranium roots, Vaccinium leaves, Quercus bark, and Hamamelis bark are all helpful in reducing diarrhea. Teas of these herbs would provide the optimal surface contact with the mucous membranes. Potassium and electrolyte replacement may be useful supportive measures. Agents that are antimicrobial to the most common intestinal pathogens include Mahonia, Hydrastis, and Artemesia. What little research has been performed on herbal agents showing efficacy against Campylobacter has been done in China and suggests that Astragalus and Atractylodes may be of value.26 When gripping in the intestines, cramping, and severe gas are a problem, Mentha, Matricaria, Dioscorrea, Viburnum, and small doses of Atropa belladonna are indicated. Agents that are soothing to the intestinal mucosa such as A vera, Glycyrrhiza, Ulmus, and Symphytum may be useful ingredients in tea and tincture formulas, promoting repair and protection of enterocytes. Nausea may be alleviated by carbonated liquids or by Mentha tea. Miso soups and vegetable broths with salt may help maintain electrolytes, as would commercial electrolyte powders such as the vitamin C powder blends. Activated charcoal and betonite clays may be offered to help absorb excessive fluids and bacterial endotoxins without artificially halting diarrhea.27,28
Some intestinal pathogens, including P aeruginosa, Staphylococcus, E coli, and Candida albicans, may form biofilms that deter the efficacy of antibiotics.29 If it comes down to a need for antibiotics, azithromycin is said to be useful for fluoroquinolone-resistant Campylobacter species. Rifaximin is a unique antibiotic that is not absorbed systemically and is reported to be useful in preventing and treating enteric infections.30 Pharmaceutical antibiotics, however, are not usually indicated, as they have not been shown to be highly effective and have been noted to foster antibiotic resistance and promote carrier states of some enteric pathogens.
 Jillian Stansbury, ND has practiced in SW Washington for nearly 20 years, specializing in women’s health, mental health and chronic disease. She holds undergraduate degrees in medical illustration and medical assisting, and graduated with honors in both programs. Dr. Stansbury also chairs the botanical medi-cine program at NCNM and teaches the core botanical curricula, a position she has held for over 18 years. In addition, Dr. Stansbury also writes and serves as a medical editor for numerous professional journals and lay publications, plus teaches natural products chemistry and herbal medicine around the country. At present she is working to set up a humanitarian service organization in Peru and studying South American ethnobotany. She is the mother of two adult children, and her hobbies include art, music, gardening, camping, international travel and studying quantum and metaphysics.
Jillian Stansbury, ND has practiced in SW Washington for nearly 20 years, specializing in women’s health, mental health and chronic disease. She holds undergraduate degrees in medical illustration and medical assisting, and graduated with honors in both programs. Dr. Stansbury also chairs the botanical medi-cine program at NCNM and teaches the core botanical curricula, a position she has held for over 18 years. In addition, Dr. Stansbury also writes and serves as a medical editor for numerous professional journals and lay publications, plus teaches natural products chemistry and herbal medicine around the country. At present she is working to set up a humanitarian service organization in Peru and studying South American ethnobotany. She is the mother of two adult children, and her hobbies include art, music, gardening, camping, international travel and studying quantum and metaphysics.
References
1. Lääveri T, Kantele A, Hakanen A, Mattila L. Traveler’s diarrhea: the most common health problem of travelers. Duodecim. 2010;126(4):403-410.
2. Darkoh C, Lichtenberger LM, Ajami N, Dial EJ, Jiang ZD, DuPont HL. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob Agents Chemother. 2010;54(9):3618-3624.
3. Teague NS, Srijan A, Wongstitwilairoong B, et al. Enteric pathogen sampling of tourist restaurants in Bangkok, Thailand. J Travel Med. 2010;17(2):118-123.
4. Fuertes PZ, Pérez-Ayala A, Pérez Molina JA, et al. Clinical and epidemiological characteristics of imported infectious diseases in Spanish travelers. J Travel Med. 2010;17(5):303-309.
5. Apelt N, Hartberger C, Campe H, Löscher T. The prevalence of norovirus in returning international travelers with diarrhea. BMC Infect Dis. 2010;10:e131.
6. Anane S, Attouchi H. Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterol Clin Biol. 2010;34(8-9):450-464.
7. Hill DR, Beeching NJ. Travelers’ diarrhea. Curr Opin Infect Dis. 2010;23(5):481-487.
8. Guttman JA, Lin AE, Li Y, et al. Gap junction hemichannels contribute to the generation of diarrhoea during infectious enteric disease. Gut. 2010;59(2):218-226.
9. Hoogerwerf WA. Role of clock genes in gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol. 2010;299(3):G549-G555.
10. Cohen D, Tobias J, Spungin-Bialik A, et al. Phenotypic characteristics of enterotoxigenic Escherichia coli associated with acute diarrhea among Israeli young adults. Foodborne Pathog Dis. 2010;7(10):1159-1164.
11. Sukhotnik I, Agam M, Shamir R, et al. Oral glutamine prevents gut mucosal injury and improves mucosal recovery following lipopolysaccharide endotoxemia in a rat. J Surg Res. 2007;143(2):379-384.
12. Rapin JR, Wiernsperger N. Possible links between intestinal permeability and food processing: a potential therapeutic niche for glutamine. Clinics (Sao Paulo).
13. Dr. Duke’s Phytochemical and Ethnobotanical Databases. http://www.ars-grin.gov/cgi-bin/duke/highchem.pl.
14. Dia A, Gautret P, Adheossi E, et al. Illness in French travelers to Senegal: prospective cohort follow-up and sentinel surveillance data. J Travel Med. 2010;17(5):296-302.
15. Malamud A, Wilson KT. Treatment of gastrointestinal infections. Curr Opin Gastroenterol. 2000;16(1):51-55.
16. Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. 2008;29(6):902-910.
17. Yismaw G, Tessema B, Mulu A, Tiruneh M. The invitro assessment of antibacterial effect of papaya seed extract against bacterial pathogens isolated from urine, wound and stool. Ethiop Med J. 2008;46(1):71-77.
18. Chang MX, Yao CY, Liu XP, Ma Z, Liu JX, Huang DY. Bacteriostasis of Rhizoma coptidis combined with trimethoprim (TMPO) [in Chinese]. Zhongguo Zhong Yao Za Zhi. 1994;19(12):748-749, 764.
19. Yang Y, Kang N, Xia H, Li J, Chen L, Qiu F. Metabolites of protoberberine alkaloids in human urine following oral administration of Coptidis rhizoma decoction. Planta Med. 2010;76(16):1859-1863.
20. Sohni YR, Kaimal P, Bhatt RM. The antiamoebic effect of a crude drug formulation of herbal extracts against Entamoeba histolytica in vitro and in vivo. J Ethnopharmacol. 1995;45(1):43-52.
21. Kageyama-Yahara N, Wang P, Wang X, Yamamoto T, Kadowaki M. The inhibitory effect of ergosterol, a bioactive constituent of a traditional Japanese herbal medicine saireito on the activity of mucosal-type mast cells. Biol Pharm Bull. 2010;33(1):142-145.
22. Soković M, Glamočlija J, Marin PD, Brkić D, Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15(11):7532-7546.
23. Fabian D, Sabol M, Domaracká K, Bujnáková D. Essential oils: their antimicrobial activity against Escherichia coli and effect on intestinal cell viability. Toxicol In Vitro. 2006;20(8):1435-1445.
24. Liao F, Huang Q, Yang Z, Xu H, Gao Q. Experimental study on the antibacterial effect of Origanum volatile oil on dysentery bacilli in vivo and in vitro. J Huazhong Univ Sci Technolog Med Sci. 2004;24(4):400-403.
25. Zhou Y, Taylor B, Smith TJ, et al. A novel compound from celery seed with a bactericidal effect against Helicobacter pylori. J Pharm Pharmacol. 2009;61(8):1067-1077.
26. Fang JY. Effect of fu-zheng qu-xie on gastric disease infected with Campylobacter pyloridis [in Chinese]. Zhong Xi Yi Jie He Za Zhi. 1991;11(3):150-152, 133.
27. Dominy NJ, Davoust E, Minekus M. Adaptive function of soil consumption: an in vitro study modeling the human stomach and small intestine. J Exp Biol. 2004;207(pt 2):319-324.
28. Klein N, Fröhlich F, Krief S. Geophagy: soil consumption enhances the bioactivities of plants eaten by chimpanzees. Naturwissenschaften. 2008;95(4):325-331.
29. Wang C. Advances in study on Traditional Chinese Medicine against biofilms [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2010;35(4):521-524.
30. Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26(1):17-25.