A Naturopathic Perspective
Emily A. Kane, ND, LAc
There’s a saying among cardiologists that rate is easier to control than rhythm. One of the problems with arrhythmias is that the anti-arrhythmic drugs are typically rife with dangerous side effects. The best known, amiodorone, works by blocking sodium and potassium flux in cardiac muscle cells. Possible side effects include lung damage, hypothyroidism, cataracts and skin discoloration.1,2 Naturopathic therapy can be very effective for mild arrhythmias. This paper will focus primarily on what I have learned about atrial fibrillation (AFib) over the years, but first I will review a few other common arrhythmias. (See Figure 1 for an example of normal sinus rhythm.)
Premature Ventricular Contractions (PVCs)
Premature ventricular contractions (PVCs) are quite common, almost always completely benign and usually “treated” adequately with patient reassurance (Figure 2). Caffeine and other stimulants tend to worsen these hard, extra beats. Typically, with PVCs the cardiac rhythm does not deviate for long from sinus rhythm, but sometimes PVCs can be frequent enough and noticeable enough, particularly in a thin patient, to cause considerable consternation. When avoidance of stimulants, which besides caffeine might include bronchodilators, is insufficient to provide relief, ½ dropperfull (about 15 drops) of Cactus grandiflorus or Selenicereus grandiflorus, daily at the same time of day, can significantly ease the frequency and severity of PVCs. Patients with PVCs regularly request refills of Cactus, and stop pursuing other avenues of relief, which is perhaps not “proof” but certainly anecdotal evidence that the therapy works well. There exists literature support that deficiencies of taurine and L-arginine can cause PVCs, as well as atrial fibrillation and premature atrial contractions (PACs). In one study of people with very frequent arrhythmias, taking 10-20 g taurine daily prevented PVCs, but not pauses, in the sinus rhythm. Adding 4-6 g L-arginine terminated the pauses. Taurine is thought to dampen epinephrine release (sympathetic activity), and L-arginine, being a nitric oxide precursor, may help create the conditions for spontaneous cardioversion.3 As with all arrhythmias, rhythm disturbances may be a manifestation of early Graves’ disease, but this is not a common etiology.4
PVCs
In a 104-pound adult female patient, PVCs were controlled by Cactus for about a decade. At menopause, her PVCs become significantly worse and she was having up to 20 PVCs per minute, for hours at a time.5 Clearly this was an untenable situation for her, despite knowing they were “benign.” Estrogen therapy helped somewhat, but caused fibroids to grow. Her thyroid function was normal. Being thin made her more sensitive to both the PVCs and the fibroid pressure. She was referred to an electrophysiologist who found that she had an extraordinary amount of ectopic electrical tissue distributed throughout the heart, including deep in the septum, which separates the 2 ventricles. She received a total of 3 electrocautery reductions of ectopic tissue, which significantly helped reduce the PVCs. However, because of the widespread nature of her ectopic tissue and the tricky location in the septum, some ectopic tissue remains and she does occasionally suffer from a run of hard PVCs. I also discovered with this patient that cardiac tonics, such as Cretaegus oxyacantha (hawthorn), were contraindicated because they made the PVCs stronger. Her heart as a pump and vascular structure have no defects – her aerobic capacity is good. She, like most patients with PVCs, simply has an electrical problem.
Paroxysmal Supraventricular Tachycardia (PSVT)
Another fairly common arrhythmia is PSVT: paroxysmal supraventricular tachycardia (Figure 3). This is slightly more common in tall people, possibly exacerbated by the elongation of the cardiac muscle. Typically, during an episode the pulse will more than double and the person will begin to feel quite fatigued, as though they just ran a marathon. These episodes tend to come on with stress, but can also be provoked by stimulants. Valsalva maneuver can terminate an episode. Also, cold face dips may help to convert PSVT into sinus rhythm by triggering the “drowning reflex” (which, incidentally, can also help break an asthma attack). In both instances, the vagus nerve is activated and electrical conduction in the heart is slowed. Nitric oxide, a vasodilator, increases with amyl nitrate, nitroglycerine, or L-arginine, and may be helpful in breaking a run of PSVT, but I have found cold face dips to be just as helpful. Homeopathic glonoine can help stop PSVT, as can rest. After several hours of conservative therapeutic attempts to restore sinus rhythm, the patient may choose to go to the emergency room, where typically IM adenosine is given, which stops the heart for around 5 seconds and allows sinus rhythm to resume. Ultimately, the patient with chronic PSVT will do best with catheter ablation, where a wire is passed to the heart through a vein at the groin, and abnormal tissue is cauterized with electrical energy.6 This typically provides definitive relief. In my opinion, the risks of long-term anti-arrhythmic drug therapy is far riskier, and much less satisfactory, than ablation performed by a skilled electrophysiologist.
PSVT
A 40-year-old, 6’ 6” man, basketball player, and new dad, had been muddling through infrequent but significant episodes of PSVT since his teen years. He would occasionally go to the ER and get a dose of IV verapamil, a calcium channel blocker, which worked, but was expensive and unpleasant, and clearly not taking care of the problem long-term. When he sought naturopathic care, the cold face dips, homeopathic glonoine, and rest worked well for many years. After the birth of his daughter, he endured a whole week in PSVT and was completely exhausted. An ER doc told him about catheter ablation. This proved to be the definitive therapy and he has not had a single episode of PSVT in 15 years.
Long QT syndrome
Long QT syndrome (Figure 4) is mostly a genetic condition that can lead to recurrent syncope and sudden death due to a ventricular tachyarrhythmia called “torsade de pointes.7 No therapy other than beta-blockers like propanolol and nadolol have been shown to keep patients out of this lethal arrhythmia, which sometimes requires an implantable defibrillator.
Complete Heart Block
Complete heart block (Figure 5) is an episodic and potentially lethal arrhythmia caused by an interruption in electrical conduction from the sinus node to the ventricles. It leads to very slow heart rates or long pauses. This third-degree heart block is also known as atrioventricular (AV) dissociation.
The one patient I have seen with this condition presented with periodic syncope with no warning whatsoever. He did not have orthostatic hypotension. He was normal weight, had good cardiac tone, and appropriate blood pressure/pulse in the office. His cardiac valves sounded great – no murmurs or bruits or evidence of valvular prolapse. He worked as a carpenter and was frequently up on ladders or driving a truck full of lumber. He was, justifiably, deeply concerned that he would black out while driving or on a ladder. The heart block was discovered on EKG. He was referred immediately for a pacemaker, and has been free of syncope since. We found out later that his sister had the same problem and she too was referred for pacemaker implantation. People with pacemakers should be advised to periodically have their pacemakers “interrogated” at a facility capable of analyzing 6-12 months’ of cardiac activity to make sure there are no occult arrhythmias.
Atrial Fibrillation (AFib)
Atrial fibrillation (AFib) is an increasingly-diagnosed condition (Figure 6). Ten percent of the US population over the age of 80 have AFib.8 The arrhythmia often originates in the pulmonary veins.9-11 All of us have the capacity to go into AFib, because we all have the “ectopic” tissue in our 4 pulmonary veins, all of which terminate in the left atrium. AFib is a condition of non-sinus rhythm, creating a sort of crazy feedback loop in the left atrium, with completely chaotic electrical activity and no effective contraction.12-14 Atrial flutter (Figure 7) is similar to AFib but involves an organized circle of electrical activation in the right atrium, often producing a regular but rapid pulse.
The major danger of AFib and flutter is an increased risk of stroke due to hemodynamic turbulence.15,16 AFib is the number one cause of strokes.17-24 Although the risks of anti-coagulation may outweigh benefits in an individual patient, across-the-board patients in AFib using anti-coagulation will generally appreciate lower morbidity and mortality from AFib and its major complication, stroke. Conventional providers’ standard of care is to place all patients in AFib on warfarin. This is an inexpensive blood thinner which certainly saves lives. However, by some accounts, the need-to-treat number for warfarin to prevent a stroke is quite high, at about 300. Basically, this means 299 people are being put at high risk for an acute hemorrhagic event in order to prevent stroke in one person.
As naturopathic physicians, we typically do not operate under a pharmaceutically-driven standard of care. We are going to assess enzymatic clot-busters, fish oil, gingko, garlic and flavonoid concentrates to reduce stroke risk. We will consider increasing magnesium, orally, IV or via Epsom salt baths. We will assess electrolyte balance because potassium may be low. Adequate Vitamin D3 saturation may reduce risk of stroke. We will work with our patient to identify stressors that can be removed or mitigated. We will make sure their thyroid function is optimal.
Sometimes AFib is episodic, with long stretches of time between episodes. If a patient presents to the ER in AFib, usually 20 mg IV diltiazem is given to slow the heart rate via the AV node. Naturopathic physicians can help reduce the incidence of AFib in the same manner that we approach all of our patients: prevention. Given that all of us could develop AFib, because the electrical fibers in the pulmonary veins exist in all of us, our job is to be aware of noxious triggers and to help our patients, as soon as possible, to develop lifestyles which avoid the known triggers. It should be no surprise that smoking, excessive alcohol consumption, and longstanding hypertension are all triggers. Hypertension causes cardiac wall thickening, which decreases compliance and provokes structural pathology in the atria. The atria stretch out, which contributes to electrical instability. Acute AFib triggers include caffeine, Ephedra, pseudoephedrine, and bronchial inhalers. These agents will also provoke other arrhythmias.
If a patient is chronically going to the ER in AFib, a more definitive solution, once again, may be ablation of the ectopic fibers.25-27 Cather ablation is widely performed for chronic AFib, as for other arrhythmias. A newer technique involves not burning, but freezing of the ectopic tissue. Some enthusiasts of cryo-ablation claim the procedure is much safer than cautery, has a lower complication rate, and a lower need for a second surgery. About 25% of patients presenting for ablation cautery to treat AFib require a second surgery. Although it is too soon to be accurate, predictions have been made that the re-do rate with cryo-ablation will be only 10%. One of the stated benefits of cryo-ablation is that this technique spares the endothelium and kills the deeper tissue where the ectopic fibers reside. One problem with both cryo and cautery is the proximity of the left atrium to the esophagus. Inadvertently burning or freezing the esophagus is almost always a lethal mistake, though fortunately very uncommon.
AFib
An 82-year-old gentleman with a long history of alcoholism developed episodic AFib and was put on amiodorone. Within 2 weeks his personality changed and he became moody and volatile. His wife, for the first time in their long marriage, was afraid of him. He also has celiac disease with concomitant pernicious anemia, which may have made the amiodorone more problematic than usual. Because of the celiac disease, he had chronic malabsorption issues beyond B12, and warfarin was untenable because it caused horrendous epithelial friability. The man complained that he looked and felt like a “walking, oozing bruise.” He was referred for catheter ablation, which was successful, and he no longer needed medical therapy for AFib.
A 91-year-old woman was discovered to have AFib at an annual wellness check. She was delightfully fit and clear-headed. Her only medicine was some low-dose thyroid support. She supplemented with vitamin C, vitamin D, and fish oil, and ate vegetables daily. Her allopath prescribed warfarin as per the standard of care. She refused because she had known several people who had died from hemorrhagic events while on warfarin. This active elder lived on the beach with a long run of stairs up to the road and didn’t want to risk being over-anti-coagulated when she had some potential for falling. I prescribed nattokinase and increased vitamin C to bowel tolerance. Unfortunately, she was spotty with compliance on the natto and suffered a stroke. She was no longer able to care for herself after the stroke and died about 6 months later in a nursing home.
I wish to thank my favorite referral electrophysiologist, Dr Lee Dolack (Swedish Hospital, and private practice in Seattle, WA) for reviewing and providing some technical corrections for this essay.
Figure 1. Normal Sinus Rhythm
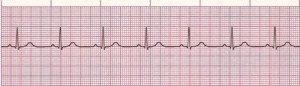
Rate: Normal (60–100 bpm); Rhythm: Regular; P Waves: Normal (upright and uniform); PR Interval: Normal (0.12–0.20 sec); QRS: Normal (0.06–0.10 sec) (Courtesy of www.rn.org)
Figure 2. Premature Ventricular Contractions
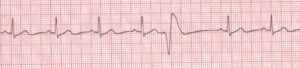
Rate: Depends on rate of underlying rhythm; Rhythm: Irregular whenever a PVC occurs; P Waves: None associated with the PVC; PR Interval: None associated with the PVC; QRS: Wide (>0.10 sec), bizarre appearance (Description, courtesy of www.rn.org)
Figure 3. Paroxysmal Supraventricular Tachycardia
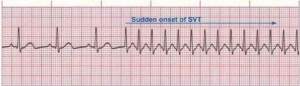
Rate: 150–250 bpm; Rhythm: Irregular; P Waves: Frequently buried in preceding T waves and difficult to see; PR Interval: Usually not possible to measure; QRS: Normal (0.06–0.10 sec) but may be wide if abnormally conducted through ventricles (Courtesy of www.rn.org)
Figure 4. Long QT Syndrome
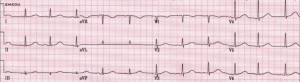
Rate: Bradycardia or tachycardia; Rhythm: Irregular; P Waves: Abnormal; QT interval: Prolonged (>450 msec); Other: Bizarre or notched T wave morphology; prominent U waves or T wave alternans. (Image, courtesy www.emedu.org)
Figure 5. Complete Heart Block
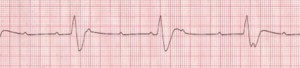
Rate: Atrial: 60–100 bpm; ventricular: 40–60 bpm if escape focus is junctional, <40 bpm if escape focus is ventricular; Rhythm: Usually regular, but atria and ventricles act independently; P Waves: Normal (upright and uniform); may be superimposed on QRS complexes or T waves; PR Interval: Varies greatly; QRS: Normal if ventricles are activated by junctional escape focus; wide if escape focus is ventricular (Courtesy of www.rn.org)
Figure 6. Atrial Fibrillation
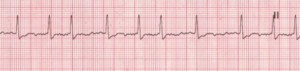
Rate: Atrial: >350 bpm; ventricular: variable Rhythm: Irregular; P Waves: No true P waves; chaotic atrial activity; PR Interval: None; QRS: Normal (0.06–0.10 sec) (Description, courtesy of www.rn.org)
Figure 7. Atrial Flutter
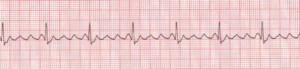
Rate: Atrial: 250–350 bpm; ventricular: variable. Rhythm: Atrial: regular; ventricular: variable; P Waves: Flutter waves have a saw-toothed appearance; some may not be visible, being buried in the QRS; PR Interval: Variable; QRS: Usually normal (0.06–0.10 sec), but may appear widened if flutter waves are buried in QRS (Description, courtesy of www.rn.org)
 Emily Kane, ND, LAc is a graduate of Bastyr University in Seattle (class of 1992), where she completed both the Naturopathic and Acupuncture/Oriental Medicine programs. Her preceptor work took place in Seattle, West Virginia, and China, with emphasis on gynecology, counseling, herbal medicine, and naturopathic manipulation. She worked as a licensed massage therapist in Seattle during her medical training for 7 years. Dr Kane maintains an active clinical practice in Juneau, Alaska, and sees patients of all ages. You can learn more about Dr Kane at http://www.DrEmilyKane.com/.
Emily Kane, ND, LAc is a graduate of Bastyr University in Seattle (class of 1992), where she completed both the Naturopathic and Acupuncture/Oriental Medicine programs. Her preceptor work took place in Seattle, West Virginia, and China, with emphasis on gynecology, counseling, herbal medicine, and naturopathic manipulation. She worked as a licensed massage therapist in Seattle during her medical training for 7 years. Dr Kane maintains an active clinical practice in Juneau, Alaska, and sees patients of all ages. You can learn more about Dr Kane at http://www.DrEmilyKane.com/.
References
Disch DL, Greenberg ML, Holzberger PT, et al. Managing chronic atrial fibrillation: a Markov decision analysis comparing warfarin, quinidine, and low-dose amiodarone. Ann Intern Med. 1994;120(6):449-457.
Clementy J, Dulhoste MN, Laiter C, et al. Flecainide acetate in the prevention of paroxysmal atrial fibrillation: a nine-month follow-up of more than 500 patients. Am J Cardiol. 1992;70(5):44A-49A.
Eby G, Halcomb WW. Elimination of cardiac arrhythmias using oral taurine and L-arginine with case histories: Hypothesis for nitric oxide stabilization of the sinus node. Med Hypotheses. 2006;67(5):1200-1204.
Krahn AD, Klein GJ, Kerr CR, et al. How useful is thyroid function testing in patients with recent-onset atrial fibrillation? The Canadian Registry of Atrial Fibrillation Investigators. Arch Intern Med. 1996;156(19):2221-2224.
Babiker FA, De Windt LJ, van Eickels M, et al. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002;53(3):709-19.
Page RL, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89(1):224-227.
Vincent GM. The long QT syndrome. Indian Pacing Electrophysiol J. 2002;2(4):127-142.
Fitzmaurice DA, Hobbs FD, Jowett S, et al. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335(7616):383.
Pritchett EL. Management of atrial fibrillation. N Engl J Med. 1992;326(19):1264-1271.
Atrial fibrillation: current understandings and research imperatives. The National Heart, Lung, and Blood Institute Working Group on Atrial Fibrillation. J Am Coll Cardiol. 1993;22(7):1830-1834.
Lip GY, Metcalfe MJ, Rae AP. Management of paroxysmal atrial fibrillation. Q J Med. 1993;86(8):467-472.
Wilber DJ. Pursuing sinus rhythm in patients with persistent atrial fibrillation: when is it too late? J Am Coll Cardiol. 2009;54(9):796-798.
European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369-2429.
Kopecky SL, Gersh BJ, McGoon MD, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317(11):669-674.
Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57(2):223-242.
Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513.
Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. JAMA. 1985;254(24):3449-3453.
Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306(17):1018-1022.
Takahashi N, Seki A, Imataka K, Fujii J. Clinical features of paroxysmal atrial fibrillation. An observation of 94 patients. Jpn Heart J. 1981;22(2):143-149.
Leong DP, Eikelboom JW, Healey JS, Connolly SJ. Atrial fibrillation is associated with increased mortality: causation or association? Eur Heart J. 2013;34(14):1027-1030.
Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J. 2013;34(14):1061-1067.
Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946-952.
Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359-364.
European Heart Rhythm Association (EHRA), European Cardiac Arrhythmia Society (ECAS), American College of Cardiology (ACC), et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4(6):816-861.
Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257-e354.
American College of Cardiology Foundation, American Heart Association, European Society of Cardiology, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2013;127(18):1916-1926.

