Understanding Risk Factors, Not Causation
Learn how much Tylenol pregnant women can safely take, what risk factors matter, and why glutathione status—not acetaminophen itself—determines safety during pregnancy.
IN THIS ARTICLE
• Key Takeaways: Tylenol Safety for Pregnant Women
• Tylenol for Pregnant Women: What You Need to Know
• The Timeline: Tylenol Introduction and Rising Neurodevelopmental Disorder Rates
• Why Glutathione Matters for Brain Development
• What the Research Shows About Tylenol and Pregnancy
• How Much Tylenol Can I Take While Pregnant? Current Usage Patterns
• How Tylenol Is Metabolized: The Three Pathways
• How Pregnancy Changes Acetaminophen Metabolism
• What Slows and Speeds Up Your Detox Enzymes
• The Toxicity of NAPQI: Why This Metabolite Is Dangerous
• Who May Be at Higher Risk? Your Individual Safety Profile
• The Postnatal Risk: Tylenol After Vaccination
• The Bottom Line: Is Tylenol Safe for Pregnancy?
• Frequently Asked Questions
• Complete References
KEY TAKEAWAYS: TYLENOL SAFETY FOR PREGNANT WOMEN
• Tylenol itself is not toxic—it only becomes problematic when your body cannot properly metabolize it through safe detoxification pathways
• The key factor is glutathione status: Glutathione acts as your body’s master antioxidant and is essential for both safely processing acetaminophen and supporting healthy fetal brain development[¹⁻²⁻³⁻⁴⁻⁵]†
• High-risk conditions: PCOS (50% lower glutathione[¹⁷⁻³⁷⁻⁴⁰]), IVF conception (70-83% depleted antioxidants[¹⁸⁻¹⁹⁻⁴¹⁻⁴⁶]), gestational blood sugar regulation issues, gallbladder dysfunction[⁵³⁻⁶⁰], and choline deficiency (89% of pregnant women[⁶¹⁻⁶³])
• 62-65% of pregnant women use Tylenol during pregnancy, with standard doses of 500-650 mg every 4-6 hours[¹⁵⁻¹⁶⁻²⁰]
• Pregnancy metabolism shifts INCREASE toxicity: 80% increase in the pathway that generates toxic NAPQI, with 43% MORE NAPQI in first trimester when fetal brain is most vulnerable, 33% LESS sulfation capacity, and glutathione depleted 36-87% throughout pregnancy[⁶⁰⁻¹⁰²⁻¹⁰⁴]
• Research shows dose-response relationships: Longer acetaminophen use correlates with higher attention/focus disorders and neurodevelopmental disorder risk, with children having highest cord blood metabolites facing 2-3x higher odds[¹¹⁻¹⁴⁻⁷⁷⁻⁷⁹]
• The toxic metabolite NAPQI crosses the placenta and depletes fetal brain glutathione at doses below those causing maternal liver toxicity[²⁴⁻²⁵⁻⁷³⁻⁷⁵⁻¹⁰⁰]
• Post-birth vulnerability continues: Increases workload on immature detoxification system. Depletion of glutathione impairs neurodevelopment. 64% of infants receive acetaminophen after vaccination[⁴⁷⁻⁴⁸]
The goal isn’t to create fear—it’s to understand that individual metabolic context matters when determining how much Tylenol is safe for pregnant women.
TYLENOL FOR PREGNANT WOMEN: WHAT YOU NEED TO KNOW
When searching “is Tylenol safe for pregnancy” or “how much Tylenol can I take while pregnant,” expectant mothers encounter alarming headlines linking prenatal acetaminophen use to neurodevelopmental disorders and attention/focus disorders. But does Tylenol cause these conditions? The evidence suggests something more nuanced—and more important for pregnant women to understand.
The key lies in understanding glutathione—glutathione acts as your body’s master antioxidant, essential for healthy fetal brain development.† Acetaminophen (Tylenol, paracetamol) metabolism directly depletes glutathione.[¹⁻⁵] The critical question is: what happens when women who already have low glutathione status take Tylenol during the critical window of fetal brain development?
The dramatic increase in neurodevelopmental disorder prevalence—from rare to affecting 1 in 36 children—points strongly to environmental factors. Tylenol use during pregnancy appears to be one piece of this larger environmental puzzle.[⁹⁰⁻⁹⁹]
THE TIMELINE: TYLENOL INTRODUCTION AND RISING NEURODEVELOPMENTAL DISORDER RATES
The temporal correlation between widespread acetaminophen use and neurodevelopmental disorder prevalence is striking:
ACETAMINOPHEN INTRODUCTION AND ADOPTION[⁸³⁻⁸⁹]
• 1955: Tylenol (acetaminophen) introduced for clinical use in the United States
• 1960s-1970s: First use in pregnancy for pain and fever management
• 1980s: Widely recommended as first-line agent for pain and fever in pregnancy
• Late 1980s-1990s: Increased use for post-vaccination symptom management in infants (replacing aspirin due to Reye’s syndrome concerns)
• 2000s-present: Routine use in newborns/infants after vaccination; 62-65% of pregnant women use acetaminophen during pregnancy
• By 9 months of age: Up to 95% of children have been exposed to acetaminophen
NEURODEVELOPMENTAL DISORDER PREVALENCE TIMELINE[⁹⁰⁻⁹⁹]
• 1960s: Prevalence estimated at 1 in 10,000
• 1980s: Prevalence rises to 1 in 2,000
• Late 1980s-1990s: Rates begin dramatic increase
• Late 1990s: Prevalence reaches 1 in 200
• 1996-2010: 269% increase in ASD prevalence (Atlanta surveillance data)
• 2021: Prevalence reaches 1 in 44 in the United States
• 1998-2018: 787% increase in recorded incidence in the UK
THE TEMPORAL PATTERN
The dramatic rise began in the late 1980s and accelerated through the 1990s and 2000s—approximately 25-30 years after acetaminophen’s introduction and widespread adoption in pregnancy, and coinciding with increased use for post-vaccination symptom management in infants.
While changes in diagnostic criteria, increased awareness, and improved screening explain part of this increase, studies suggest that environmental exposures may also contribute to a genuine rise in prevalence. The timing aligns with the period when acetaminophen became ubiquitous in pregnancy and early childhood—affecting both prenatal brain development and postnatal exposures during critical developmental windows.
This temporal correlation does not prove causation, but it supports the hypothesis that acetaminophen may be one of multiple environmental factors contributing to rising neurodevelopmental disorder rates, particularly in metabolically vulnerable populations unable to safely detoxify the drug.
WHY GLUTATHIONE MATTERS FOR BRAIN DEVELOPMENT†
Glutathione acts as your body’s master antioxidant and primary mechanism for supporting developing tissue’s healthy response to oxidative stress. During pregnancy, adequate glutathione levels are essential for proper brain development.[¹⁻⁵]†
GLUTATHIONE’S CRITICAL ROLES[¹⁻⁵]†
• Supports the developing brain’s healthy response to oxidative stress
• Supports healthy methylation (controls gene expression)
• Regulates homocysteine levels (elevated levels risk neural tube defects)
• Enables proper epigenetic programming
• Supports a healthy immune response within the brain and spinal chord and normal microglial activation
THE CONNECTION TO NEURODEVELOPMENTAL DISORDERS
Children with neurodevelopmental disorders consistently show low glutathione levels, elevated homocysteine, impaired methylation capacity, oxidative stress markers, and mitochondrial dysfunction.[⁶⁻⁸]
Low maternal glutathione during pregnancy is associated with preeclampsia, HELLP syndrome, intrauterine growth restriction, fetal brain inflammation, and neurodevelopmental disorders.[²⁻³⁻⁶⁻⁹⁻¹⁰]
WHAT THE RESEARCH SHOWS ABOUT TYLENOL AND PREGNANCY
Several large observational studies identify associations between prenatal acetaminophen use and neurodevelopmental outcomes:
• A 2021 meta-analysis of 73,881 mother-child pairs found children prenatally exposed to acetaminophen were 19% more likely to have symptoms associated with neurodevelopmental conditions and 21% more likely to have attention/focus symptoms.[¹¹]
• A 2020 study measuring cord blood biomarkers found dose-response relationships with attention/focus disorders and neurological disorders risk.[¹²]
• A 2018 meta-analysis of 132,738 mother-child pairs found associations that increased with longer exposure duration.[¹³]
• A 2024 evaluation reviewed 46 studies, with higher-quality studies more likely to show positive associations.[¹⁴]
HOW MUCH TYLENOL CAN I TAKE WHILE PREGNANT? CURRENT USAGE PATTERNS
• 62-65% of pregnant women use acetaminophen at least once[¹⁵⁻¹⁶]
• 6% use it weekly or more often[¹⁵⁻¹⁶]
• Standard dose: 500-650 mg every 4-6 hours as needed[¹⁵⁻¹⁶⁻²⁰]
• 58% use it for fewer than 10 days; 9% use it for 45+ days[¹⁶]
These “safe” therapeutic doses can significantly deplete glutathione in women who already have low baseline levels.[²¹⁻²³] Healthy individuals can tolerate up to 4g/day with only modest glutathione decrease, but individuals with compromised glutathione experience significant depletion even at therapeutic doses.
THE MATH THAT MAKES YOU STOP AND THINK
• Neurodevelopmental disorder rate: 1 in 36 children
• 3.6 million births per year in the USA
• 1:36 = 100,000 children diagnosed annually
• 65% of pregnant women take acetaminophen (2.3 million women annually)
• At least 10% have compromised glutathione (PCOS, IVF, poor diet, chemical exposure, genetics)
• That’s 200,000+ pregnancies per year where acetaminophen depletes the molecule needed for healthy brain development
If even half of those 200,000 vulnerable pregnancies result in developmental issues, you’ve accounted for a significant portion of the affected population.
HOW TYLENOL IS METABOLIZED: THE THREE PATHWAYS
When you take acetaminophen, your liver processes it through three primary pathways:[²⁶⁻²⁷⁻⁶⁰⁻⁷⁰⁻⁷²]
1. GLUCURONIDATION – SAFE PATHWAY (UGT1A6 enzyme)
• 50-60% of acetaminophen in healthy non-pregnant adults
• Attaches glucuronic acid to make it water-soluble for urinary excretion
• Safe, non-toxic pathway
• INCREASES during pregnancy: 6% in first trimester, rising to 19% by third trimester[⁶⁰]
• Severely underdeveloped in fetuses/newborns
2. SULFATION – SAFE PATHWAY (SULT enzymes)
• 30-35% of acetaminophen in healthy non-pregnant adults
• Attaches sulfate groups for safe elimination
• Non-toxic pathway
• DECREASES 33% during pregnancy (contrary to previous assumptions)[⁶⁰]
• Limited capacity—easily saturated with repeated doses
• Predominant pathway in fetuses/children (60-70%) but quickly overwhelmed
3. OXIDATION – TOXIC PATHWAY (CYP2E1 enzyme)
• Only 5-10% of acetaminophen in healthy non-pregnant adults
• INCREASES by 80% during pregnancy[⁶⁰]
• Creates NAPQI (N-acetyl-p-benzoquinone imine)—the toxic metabolite
• NAPQI formation 43% HIGHER in first trimester[⁶⁰]
• NAPQI must be immediately neutralized by glutathione
• When glutathione is depleted, NAPQI causes cellular damage
• Crosses the placenta and damages fetal brain
HOW PREGNANCY CHANGES ACETAMINOPHEN METABOLISM
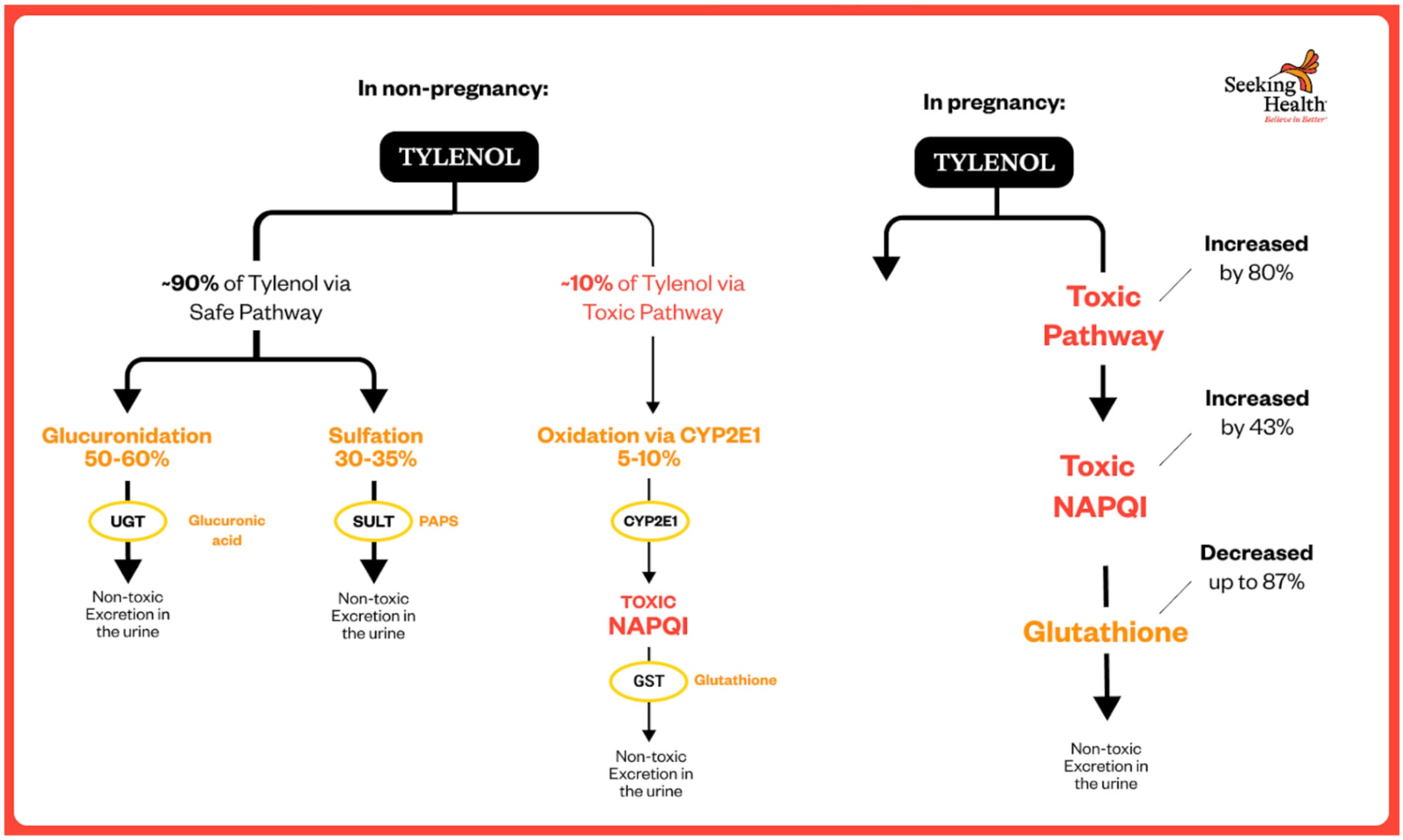
METABOLIC PATHWAY CHANGES DURING PREGNANCY[⁶⁰⁻¹⁰⁰⁻¹⁰¹]
Human pharmacokinetic studies reveal dramatic shifts in how pregnant women metabolize acetaminophen:
Oxidation to NAPQI (Toxic Pathway) INCREASES by 80%
The Brookhuis et al. 2021 study published in Pharmaceutics used physiologically-based pharmacokinetic (PBPK) modeling in pregnant women and found:
Just before giving birth, the clearance level (CL) of acetaminophen via the toxic pathway was “1.8-fold higher than shortly after delivery. This 80% increase, observed in third-trimester pregnant women was estimated to occur throughout the entire pregnancy.”[⁶⁰]
This means **43% MORE of the toxic NAPQI metabolite is produced** during pregnancy compared to non-pregnant women.
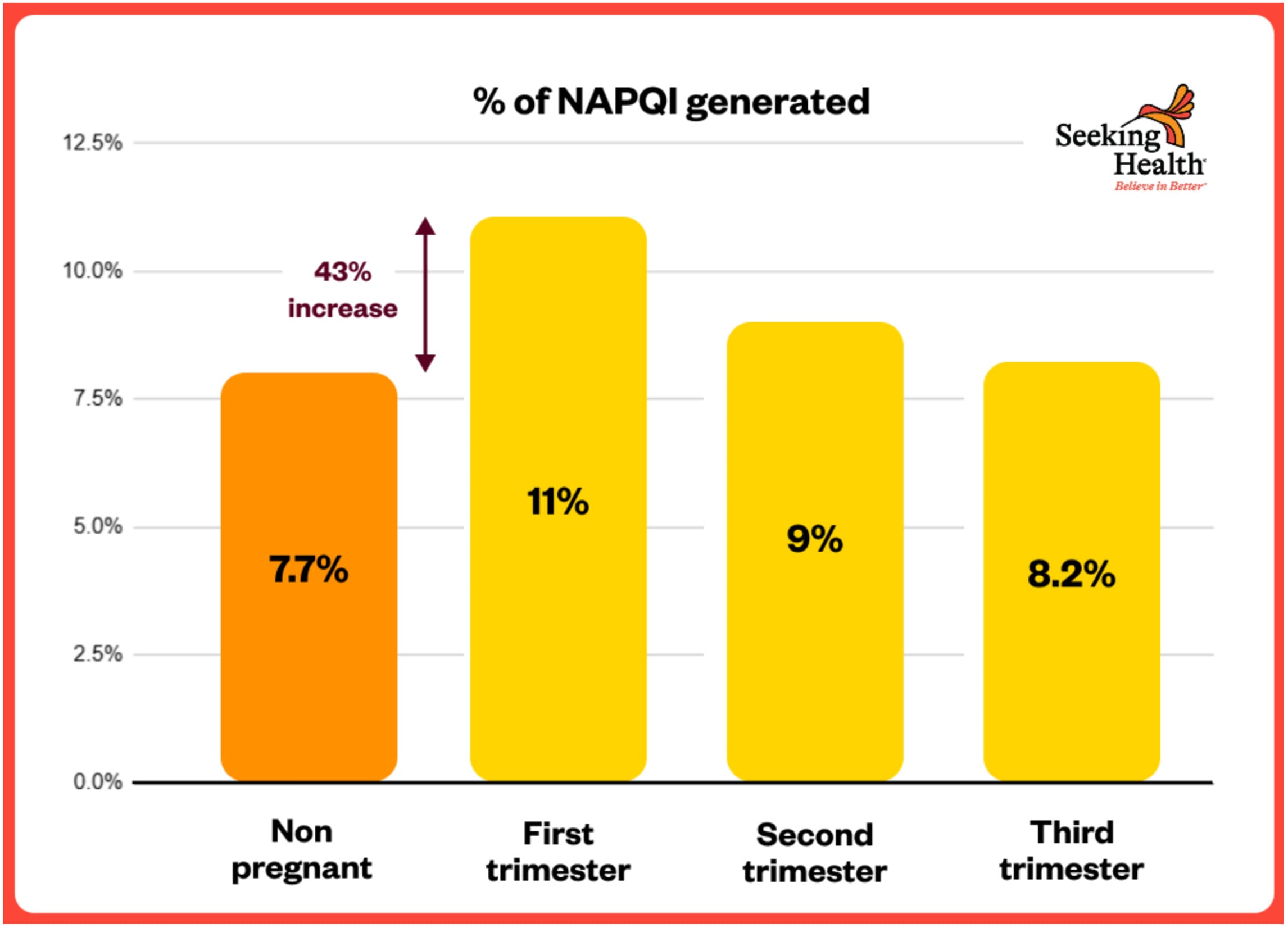
NAPQI Formation Highest in FIRST Trimester – 43% HIGHER
The same Brookhuis 2021 study found:
“The estimated molar dose fraction of NAPQI was the highest in the first trimester (median (IQR): 11 (9.1–13.4%), followed by the second (9.0 (7.5–11%)) and third (8.2 (6.8–10.1)) trimester compared with non-pregnant women (7.7 (6.4–9.4%)).”[⁶⁰]
**This represents a 43% INCREASE in toxic NAPQI formation in the first trimester** (11% vs 7.7%)—precisely when the fetal brain is most vulnerable and undergoing critical early development.
Sulfation (Safe Pathway) DECREASES 33%
The Brookhuis 2021 data showed:
“The estimated molar dose fraction of acetaminophen-sulphate decreased with the duration of pregnancy (24.2 (22.3–26.2%), 21.5 (19.9–23.1%) and 20.7 (19.1–22.4%) in the first, second and third trimester, respectively) compared with non-pregnant women (31.1 (29.0–33.3%)).”[⁶⁰]
**This is a 33% DECREASE in the safe sulfation pathway by third trimester** (20.7% vs 31.1%).
Glucuronidation Increases BUT Is Insufficient
While glucuronidation (the other safe pathway) does increase during pregnancy:
This increase in glucuronidation CANNOT compensate for the simultaneous:
• 33% decrease in sulfation (another safe pathway)
• 80% increase in oxidation to toxic NAPQI
**The Result:** When both safe pathways become saturated or overwhelmed, significantly more acetaminophen is forced through the toxic CYP2E1 pathway, creating 43% more NAPQI—at precisely the time when glutathione reserves are most depleted.
GLUTATHIONE DEPLETION DURING PREGNANCY[¹⁰²⁻¹⁰⁵]
Multiple human studies confirm that glutathione levels progressively DECREASE throughout pregnancy—creating maximum vulnerability when combined with increased NAPQI production.
Progressive Depletion Across Trimesters
A 2024 study measuring glutathione across all three trimesters found:[¹⁰²]
“The current study shows there was a significant difference in serum glutathione levels in the first, second and third trimester (sub groups) when compared to control group (48.21 ±1.13 pg./ml), (34.01 ±1.02 pg./mL), (9.76 ±0.34 pg./mL) vs. (75.24 ±1.29 pg./mL), (P≤0.01).”
This represents:
• **36% LOWER glutathione in first trimester** (48 vs 75 pg/mL)
• **55% LOWER in second trimester** (34 vs 75 pg/mL)
• **87% LOWER in third trimester** (9.76 vs 75 pg/mL)
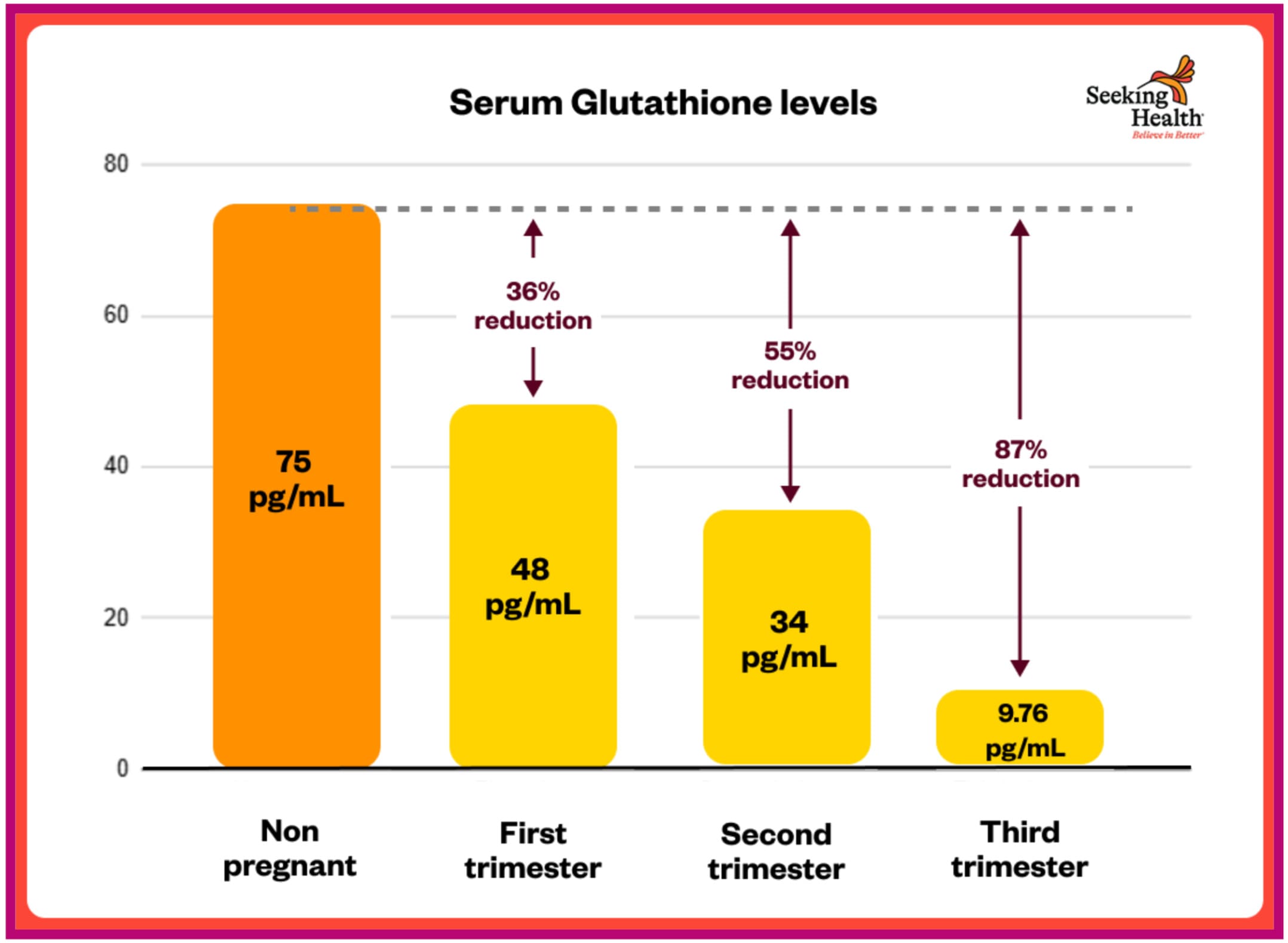
The study concluded: “The decrease in antioxidant levels and increase in malondialdehyde (MDA) level [an indicator of oxidative stress] lead us to conclude that pregnancy as a situation produces a huge amount of oxidant so reduces the capability of the body to overcome its effect leading to oxidative stress.”[¹⁰²]
>>> Glutathione Depletion in Normal Pregnancy <<<
A separate study confirmed: “It was found that the glutathione levels in second trimester of pregnancy were found to be less than non-pregnant women” and “Serum glutathione levels are significantly lower in pregnant women than non-pregnant women. (p <0.0001).”[¹⁰³]
Further Depletion in Pregnancy Complications
Kharb 2000 found: “In preeclampsia, maternal total glutathione levels were lower than in normal pregnancy (P<0.001). Also, diabetic preeclamptics showed low total glutathione levels as compared to preeclampsia (P<0.05) and control (P<0.001). These findings indicate decreased detoxificating or free radical scavenging capacity in pregnancies complicated by preeclampsia and diabetes.”[¹⁰⁴]
THE PERFECT STORM: PREGNANCY CREATES MAXIMUM VULNERABILITY
The combination of these metabolic changes creates unprecedented risk:
1. **↑ 80% INCREASE in the toxic NAPQI pathway** (43% more NAPQI generated in first trimester)
2. **↓ 33% LESS sulfation capacity** (safe detox pathway decreases)?
3. **↓ 36-87% LOWER glutathione** (needed to neutralize NAPQI)
4. **Fetal brain highly vulnerable** (immature detox enzymes, high metabolic demands)
This is why therapeutic doses considered “safe” in non-pregnant women can be toxic during pregnancy—especially in metabolically vulnerable populations with already-compromised glutathione status.
THE TOXICITY OF NAPQI: WHY THIS METABOLITE IS DANGEROUS
Acetaminophen itself is not toxic. It only becomes toxic when metabolized into NAPQI. The problem arises during pregnancy when the toxic oxidation pathway increases by 80%, producing 43% more NAPQI in the first trimester—at precisely the time when glutathione is depleted 36-87%.[⁶⁰⁻¹⁰²⁻¹⁰⁴]
NAPQI CROSSES THE PLACENTA AND DAMAGES THE FETUS[²⁴⁻²⁵⁻¹⁰⁰]
The Mian et al. 2020 study confirmed:[¹⁰⁰]
• NAPQI crosses the placenta
• Fetal liver has severely limited detoxification capacity
• Demonstrates direct fetal exposure to toxic metabolite
WHAT NAPQI DOES[⁷³⁻⁷⁵]
• Directly binds to and damages cellular proteins
• Depletes glutathione rapidly
• Generates oxidative stress which damages lipids, proteins, and DNA
• Triggers cell death (apoptosis)
• Damages mitochondria, disrupting energy production
NAPQI DEPLETES FETAL BRAIN GLUTATHIONE AT DOSES BELOW MATERNAL LIVER TOXICITY
ESSENTIAL POINT: NAPQI depletes fetal brain glutathione and induces oxidative stress and cell death in neural tissue at doses below those causing maternal liver toxicity.[⁷⁵] The developing brain has limited antioxidant defenses and high metabolic demands, making it especially susceptible.
This is why a pregnant woman can feel fine while her fetus is experiencing glutathione depletion and oxidative brain damage.
WHY LOW GLUTATHIONE IS DEVASTATING FOR THE DEVELOPING BRAIN
When NAPQI binds to glutathione, it reduces capacity for glutathione to detoxify other harmful chemicals in the developing brain, such as:
#1 Hydrogen Peroxide
Glutathione normally converts hydrogen peroxide into water. In the developing brain, hydrogen peroxide:
• Kills immature neurons at concentrations less toxic to mature neurons
• Disrupts the blood-brain barrier
• Impairs visual system development and brain connectivity
#2 Formaldehyde
Naturally produced in the body but increased by environmental exposure (new clothes, carpets, construction materials). In the developing brain, formaldehyde:
• Disrupts learning and memory
• Causes oxidative stress and DNA damage
• Promotes inflammation and cell death
• Disrupts neurotransmitter systems
#3 Toxic Metals
Mercury and arsenic are well-established neurotoxicants. In the developing brain:
• Mercury (methylmercury from fish) causes dose-dependent deficits in IQ, memory, attention, language
• Arsenic exposure (rice, drinking water) linked to cognitive deficits, lower IQ, behavioral issues
• First trimester is particularly vulnerable
#4 Homocysteine
Maternal homocysteine increases with insufficient folate and vitamin B12. High homocysteine during pregnancy is associated with:
• Neural tube defects
• Delayed brain maturation
• Cognitive deficits
WHY THE DEVELOPING BRAIN IS MORE VULNERABLE
• Higher metabolic activity: Rapid growth generates more reactive oxygen species
• Lower protective enzymes: Less capacity to detoxify hydrogen peroxide
• Immature blood-brain barrier: More permeable to chemicals and toxins
• Underdeveloped myelin: Neurons lack fatty protective coating (not fully developed until at least age 3)
• Underdeveloped detoxification: Fetal and newborn liver capacity severely limited
THE LINK TO NEURODEVELOPMENTAL DISORDERS
Attention/Focus Disorders – Most Robust Evidence[⁷⁷⁻⁷⁹]
• Clear dose-response relationship: more exposure = higher risk
• Cord blood biomarkers of NAPQI detoxification: 2–3-fold higher odds of ADHD
Neurodevelopmental Spectrum Disorders[⁷⁷⁻⁷⁹⁻⁸¹]
• Less consistent than Attention/Focus disorders
• Higher cord plasma metabolite levels associated with increased odds in some cohorts
• Higher-quality studies more likely to show positive associations
Other Outcomes[⁸¹⁻⁸²]
• Sleep and behavioral problems in exposed toddlers
• Slight increase in intellectual disability risk
**THE DOSE-RESPONSE PATTERN[⁷⁷⁻⁷⁹]**
• Short-term use (<1 week): Minimal to modest risk increase
• Longer duration (multiple weeks): Progressively higher risk
• Frequent use (weekly+): Highest risk
• Higher cord blood metabolites: 2-3x higher odds of attention & focus/neurodevelopmental concerns
This is exactly what you’d expect if NAPQI is the driver: more acetaminophen → more NAPQI → more glutathione depletion → more oxidative damage → higher neurodevelopmental risk.
RISK FACTORS FOR TYLENOL DURING PREGNANCY
DIETARY RISK FACTORS
• **Low sulfur amino acid intake** – Vegetarian/vegan diets without adequate protein, low eggs/poultry/fish/cruciferous vegetables
• **Protein-energy malnutrition** – Insufficient calories, fasting, inadequate protein
• **Micronutrient deficiencies** – Low vitamins C and E (impair glutathione recycling), selenium, B vitamins (B6, B12, folate)
METABOLIC AND HEALTH CONDITIONS
Polycystic Ovary Syndrome (PCOS) – Major Concern (5-10% of women)[¹⁷⁻³⁷⁻⁴⁰]
• 50% lower glutathione levels
• Significantly increased oxidative stress
• 3-4x higher risk of preeclampsia, 3x higher risk of gestational diabetes—both further deplete glutathione
• Critical dose concern: With 50% lower baseline glutathione, the “safe” dose is effectively cut in half
Other metabolic conditions that reduce glutathione status:
• Blood sugar dysregulation – Gestational blood sugar issues, pre-existing blood sugar issues, high-sugar diet
• Chronic infections – Viral (EBV, CMV), bacterial, chronic inflammatory conditions
• Pre-existing liver conditions – Fatty liver, chronic hepatitis
• Obesity and metabolic syndrome – Chronic inflammation, impaired glutathione synthesis
ENVIRONMENTAL EXPOSURES
• **Chlorine** – Tap water, pools, hot tubs
• **Formaldehyde and VOCs** – New clothing (unwashed), new carpets/flooring, remodeled homes, new furniture, nail salons, hair salons
• **Other chemicals** – Pesticides, heavy metals, air pollution, occupational exposures, cleaning products
LIFESTYLE FACTORS
• **High-intensity exercise** – Exhaustive exercise can acutely deplete glutathione
• **Chronic stress and inadequate sleep** – Increases oxidative stress, depletes glutathione
GENETIC FACTORS
Variants in CBS, CYP2E1, glutathione pathway genes, MTHFR, GSTM1/GSTT1 deletions, UGT enzyme polymorphisms
MEDICATION AND SUBSTANCE USE
Chronic alcohol, CYP2E1-inducing drugs, medications burdening glutathione systems, multiple medications competing for glucuronidation, estrogen-containing contraceptives (prior use), fertility drug use
PREGNANCY-SPECIFIC FACTORS
Gallbladder Dysfunction (10% of pregnant women)[⁵³⁻⁶⁰⁻¹⁰⁶⁻¹⁰⁷]
• Up to 10% develop gallstones or biliary sludge
• Intrahepatic cholestasis of pregnancy (ICP): 0.4-10% of pregnancies (up to 5.6% in Latina women)
• Sluggish gallbladder function impairs hepatic clearance
!!! CRITICAL: FATAL OUTCOMES DOCUMENTED AT THERAPEUTIC DOSES !!!
Gao et al. 2022 reported the first case of a pregnant woman with intrahepatic cholestasis who died from liver failure after taking THERAPEUTIC/NORMAL doses of acetaminophen: “Hepatic Failure With Fatal Outcome During Pregnancy Following Administration of a Single Therapeutic Dose of Acetominophen.”[⁵⁸]
This case represents the FIRST documented fatal outcome from therapeutic-dose acetaminophen in pregnancy, demonstrating that standard doses can be lethal in metabolically vulnerable pregnant women.
Liver Transplants Required at Supratherapeutic Doses
Thornton & Minns 2012 documented:[¹⁰⁶]
• 22-year-old pregnant woman
• Took 8-9 grams/day for 10-14 days (vs 4g/day maximum recommended)
• Developed fulminant hepatic failure requiring liver transplant
• Fetal demise occurred 2 weeks post-transplant
A second liver transplant case (2013):
• 22-year-old, 19 weeks pregnant
• Took approximately 6 grams daily for 2 weeks for toothache
• Developed fulminant hepatic failure requiring transplantation
• Fetus expired
These cases demonstrate that doses moderately above recommended limits—which might be tolerated in non-pregnant women—can cause catastrophic liver failure requiring transplantation during pregnancy, with fetal death as a common outcome.
Choline Deficiency (89% of pregnant women)[⁶¹⁻⁶³]
• Only 11% of pregnant women meet recommended choline intake
• Even worse for vegetarian/vegan diets (only 7% adequate)
• Choline essential for liver function, methylation, and phospholipid synthesis
• Deficiency increases susceptibility to hepatic injury
IVF/Assisted Reproductive Technology (2-5% of births)[¹⁸⁻¹⁹⁻⁴¹⁻⁴⁶]
Women who conceive via IVF experience dramatic antioxidant depletion:
• 70% reduction in plasma antioxidant capacity (3.4-fold lower than natural conception)
• 83% reduction in follicular fluid antioxidant capacity (6-fold lower)
• 10-30% decrease in total antioxidant activity after ovarian stimulation
• 15-25% decrease in vitamins E, C, carotenoids during IVF cycle
• 50-100%+ increase in oxidative stress markers
• Lower critical trace elements (copper, iron, zinc, magnesium)
• Reduced glutathione S-transferase activity in follicular fluid
Critical dose concern: With 70-83% reduced antioxidant capacity, even a single 500 mg dose represents a much greater burden. With over 5 million babies born via IVF globally (half in past 6 years), this represents a large and growing high-risk population.
**Other pregnancy-specific risk factors:** Preeclampsia, gestational blood sugar issues, gestational high blood pressure, multiple gestations, hyperemesis gravidarum, advanced maternal age, history of pregnancy complications
THE POSTNATAL RISK: TYLENOL AFTER VACCINATION
If prenatal acetaminophen creates vulnerability during fetal brain development, post-vaccination acetaminophen in infants may represent the final burden on an already compromised system.
HOW COMMON IS POST-VACCINATION TYLENOL USE?
• 64% of infants receive acetaminophen within 48 hours after vaccination[⁴⁷]
• 11% receive it prophylactically (before vaccination)[⁴⁷]
• Acetaminophen used 2.6x more frequently than ibuprofen[⁴⁷]
• Oral analgesics used in 81% of practices during injection, 89% post-injection[⁴⁸]
WHY NEWBORNS ARE UNIQUELY VULNERABLE
**Loss of Maternal Protection:** At birth, the newborn loses maternal metabolic protection and must handle acetaminophen using its own immature enzyme systems.[²⁴⁻²⁵⁻⁴⁹⁻⁵⁰]
Immature Detoxification Capacity:[⁴⁹⁻⁵²]
• Glucuronidation severely underdeveloped (primary adult pathway barely functions in newborns)
• Sulfation predominates but easily saturated
• Limited glutathione reserves (especially in preterm/sick infants)
• Impaired NAPQI detoxification
When sulfation is saturated, more acetaminophen is forced through the oxidative pathway to NAPQI—when glutathione reserves are least able to handle it.
THE COMPOUNDING EFFECT
**Before Birth:** Mother has compromised glutathione → takes acetaminophen → developing fetal brain exposed to NAPQI → fetus cannot effectively detoxify → impaired neurodevelopment and neuronal damage
**After Birth:** Baby loses maternal protection → immature detoxification systems → low glutathione → receives acetaminophen at 2, 4, 6, 12 months with vaccinations → each dose further depletes limited glutathione → cumulative burden may exceed threshold for healthy neurodevelopment → neuronal damage and regression from previously achieved milestones may occur
THE MATH THAT CONNECTS PRENATAL AND POSTNATAL EXPOSURE
• 200,000+ pregnancies per year involve acetaminophen use in women with compromised glutathione
• Of those 200,000 vulnerable prenatal exposures, 64% of infants will receive acetaminophen within 48 hours of first vaccination
• That’s approximately 128,000 infants per year with vulnerable prenatal exposure AND postnatal acetaminophen
• 100,000 children diagnosed with neurodevelopmental disorders annually
Many receive acetaminophen repeatedly—after vaccinations at 2, 4, 6, and 12-15 months. Each dose represents another hit on an already compromised system.
This isn’t about vaccines causing neurodevelopmental disorders. This is about a common medication practice that may represent one exposure too many for infants already vulnerable from prenatal exposures and maternal metabolic dysfunction.
THE BOTTOM LINE: IS TYLENOL SAFE FOR PREGNANCY?
The dramatic rise in neurodevelopmental disorder prevalence points to environmental factors playing a significant role. Acetaminophen use during pregnancy—and after birth in infancy—appears to be one environmental factor among many that can interact with underlying vulnerabilities.
The key understanding: **Acetaminophen itself is not toxic. It only becomes toxic when the body cannot properly metabolize it through safe detoxification pathways and instead converts it into the toxic metabolite NAPQI.**
The evidence suggests acetaminophen doesn’t cause neurodevelopmental disorders in babies born to women with robust detoxification systems. However, in individuals with compromised glutathione status, impaired detoxification pathways, poor methylation, genetic variants, or multiple environmental stressors, the body generates more NAPQI—the toxic metabolite that crosses the placenta, depletes fetal brain glutathione, and damages developing neurons.
KEY TAKEAWAYS
1. **The fetus cannot detoxify acetaminophen effectively** and relies entirely on maternal metabolism. Fetal metabolism uses sulfation pathways that become saturated, shunting more drug to the toxic NAPQI pathway.[²⁴⁻²⁵⁻¹⁰⁰]
2. **NAPQI is neurotoxic:** NAPQI crosses the placenta, depletes fetal brain glutathione, generates oxidative stress, damages mitochondria, and triggers cell death in neural tissue at doses below those causing maternal liver toxicity. Children with highest cord blood levels of NAPQI-related metabolites face 2-3x higher odds of attention/focus disorders.[⁷³⁻⁷⁵⁻¹⁰⁰]
3. **Pregnancy INCREASES acetaminophen toxicity:** The Brookhuis 2021 study demonstrates that pregnancy causes an 80% increase in the toxic CYP2E1 pathway that generates NAPQI, 43% higher NAPQI in first trimester when fetal brain is most vulnerable, and 33% decrease in safe sulfation pathway—while glutathione simultaneously depletes by 36-87% throughout pregnancy.[⁶⁰⁻¹⁰²⁻¹⁰⁴]
4. **Even women who feel fine may have fetuses experiencing damage:** The pregnancy-induced shift toward oxidation (↑80%) combined with decreased sulfation (↓33%) and depleted glutathione (↓36-87%) creates maximum vulnerability. A mother feeling well doesn’t mean her fetus isn’t experiencing glutathione depletion and oxidative brain damage.[⁶⁰⁻¹⁰²]
5. **Fatal outcomes documented at therapeutic doses:** Gao et al. 2022 reported the first fatal case—a pregnant woman with intrahepatic cholestasis died from liver failure after therapeutic acetaminophen doses. Multiple liver transplant cases documented at supratherapeutic doses with fetal death.[⁵⁸⁻¹⁰⁶]
6. **Dose-response relationship supports causation:** Clear dose-response patterns—longer acetaminophen use correlates with higher attention/focus disorder risk, with strongest evidence linking prenatal NAPQI exposure to attention and behavioral disorders.[⁷⁷⁻⁷⁹]
7. **Multiple risk factors compound vulnerability:** Low sulfur amino acid intake, blood sugar dysregulation, chronic infections, chemical exposures, genetic variants, IVF conception (70-83% depleted antioxidants), PCOS (50% lower glutathione), gallbladder dysfunction (10%), and choline deficiency (89%) severely compromise glutathione status and detoxification pathways.[¹⁷⁻¹⁹⁻³⁷⁻⁴⁶⁻⁶¹⁻⁶³]
8. **The numbers align:** 200,000+ vulnerable pregnancies per year involving acetaminophen exposure and 100,000 neurodevelopmental disorder cases annually (plus hundreds of thousands of attention/focus disorder cases).
9. **Postnatal exposure adds cumulative burden:** 64% of infants receive acetaminophen after vaccination, and for those already vulnerable from prenatal exposures, this may represent “the last straw” exceeding metabolic capacity.[⁴⁷⁻⁴⁸]
WHAT SLOWS AND SPEEDS UP YOUR DETOX ENZYMES
Even if you don’t have genetic variations in detoxification enzymes, your lifestyle, diet, medications, and environmental exposures can significantly impact how efficiently your body processes acetaminophen.
GLUCURONIDATION (UGT1A6 Enzyme) – THE SAFE PATHWAY
What SLOWS UGT1A6 (Makes It “Dirty”):
Environmental Chemicals:
• Persistent organochlorine pollutants (PCBs, DDT, bisphenol A/BPA, dioxins)
• Xenobiotic compounds in fabric dyes, hair dyes, skin lightening products
• Certain pesticides and herbicides
• Polyaromatic hydrocarbons (PAHs) from grilling, charring, barbecuing, deep-frying
• Produce grown near highways or pollution sources
Lifestyle Factors:
• Advancing age (naturally declines)
• Females have lower expression than males
• Inflammation and infections (especially LPS from bacterial infections and leaky gut)
• Chronic stress
Foods:
• Synthetic food dyes (halogenated xanthene food dyes, phloxine, erythrosine, rose bengal)
• High-temperature cooked foods (burnt toast, charred meats, deep-fried foods)
• Cured/smoked deli meats, bread from wood-fired ovens
Medications:109
• NSAIDs (ibuprofen, naproxen)
• Aspirin
• Acetaminophen itself (when used repeatedly)
• Glimepiride (type II diabetes medication) is a strong inhibitor of UGT1A6
• Tamazapan (Bebzodiazapine, sleeping medication)
• Mefenamic acid (pain relief for primary dysmenorrhea)
• Ketoconazole (antifungal treatment for systemic fungal infections)
• Itraconazole (antifungal treatment for both systemic and superficial fungal infections)
• Verapamil (angina medication)
• Ritonavir (antiretroviral agents for HIV-1 infection)
Herbs (worse when combined with medications):
• Milk thistle (Silybum marianum)
• Astragalus (Astragali radix)
• St. John’s wort (Hypericum perforatum)
• Saw palmetto (Serenoa repens)
• Cranberry
• High-coumarin herbs: wormwood, mullein, sweet clover, dong quai, peppermint, spearmint
What SPEEDS UP UGT1A6 (Keeps It “Clean”):
Foods:
• Cruciferous vegetables (broccoli, kale, cauliflower, Brussels sprouts)
• Onions and garlic (high in quercetin and sulfur compounds)
• High-quality organic coffee (increases glucuronidation)
• Green tea, rooibos tea, honeybush tea, dandelion tea
• Rosemary and lemon juice (especially when roasting)
Cooking Methods:
• Braising, stewing, slow-cooking (lower, indirect heat)
• Marinating meats 4+ hours before grilling (reduces damaging polyaromatic hydrocarbon (PAH) formation)
Supplements†:
• Calcium-D-glucarate
• Quercetin (paradoxically supports despite technical slowing)
• Curcumin
• Vitamin C, vitamin E (antioxidants support a healthy response to oxidative stress)
• Glutathione or N-acetylcysteine (NAC)
SULFATION (SULT Enzymes) – THE SAFE PATHWAY
What SLOWS Sulfation (Makes It “Dirty”):
Dietary Deficiencies:
• Low sulfur amino acid intake (vegetarian/vegan diets without adequate protein)
• Inadequate protein intake
• Low consumption of eggs, poultry, fish, cruciferous vegetables
Nutrient Depletions:
• Molybdenum deficiency (needed for sulfite oxidase)
• Vitamin B6 deficiency
• Magnesium deficiency
Environmental Exposures:
• Sulfite-containing foods and preservatives (wines, dried fruits, processed foods)
• High toxic metal burden (depletes sulfur reserves)
Medications:108
• Mefenamic acid (pain relief for dysmenorrhea) strongly inhibits SULT1A1
• NSAIDs (nonsteroidal anti-inflammatory drugs) such as nimesulide, meclofenamate, piroxicam, aspirin, ibuprofen, and salicylic acid (aspirin)
• Clomiphene (used to induce ovulation in women desiring pregnancy), including those with polycystic ovary syndrome, amenorrhea-galactorrhea syndrome, psychogenic amenorrhea, and certain cases of secondary amenorrhea.
• Danazol (treatment for endometriosis)
• Spironolactone (heart failure and hypertension medication)
• Cyclizine and Dimenhydrinate (treatment for nausea, vomiting, motion sickness, and vertigo).
• Chlorpheniramine (antihistamine, hay fever medication)
Herbs (worse when combined with medications):110
• Quercetin and resveratrol (wine and red berries)
• ECGC (found in green tea)
• Grape seed
• St. John’s wort (Hypericum perforatum)
• Milk thistle (Silybum marianum)
• Gingko biloba leaf
• Gymnema (type 2 diabetes, metabolic syndrome and weight management)
• Banaba (medicinal leaf extracts). Antihyperglycemic used for diabetes and metabolic syndrome
• Astragalus (Astragali radix)
• Turmeric spice (curcumin)
• Fruit juces: grapefruit juice, orange juice
• Teas: green tea, black tea and oolong tea
Genetic Factors:
• SUOX gene slow variants (sulfite oxidase deficiency)
• CBS gene slow variants (can impair sulfur metabolism)
What SPEEDS UP Sulfation (Keeps It “Clean”):
Foods:
• High-sulfur foods: eggs, garlic, onions, cruciferous vegetables
• Quality animal protein: grass-fed beef, pasture-raised poultry, wild-caught fish
• Alliums: garlic, onions, leeks, shallots
• Brassicas: broccoli, cabbage, kale, Brussels sprouts, cauliflower
Supplements†:
• MSM (methylsulfonylmethane)
• Taurine
• Alpha-lipoic acid
• Molybdenum
• Vitamin B6
• Magnesium
• N-acetylcysteine (NAC)
CYP2E1 ENZYME – THE TOXIC PATHWAY
What SPEEDS UP CYP2E1 (INCREASES Toxic NAPQI Production):
This is the enzyme you want to SLOW DOWN during pregnancy because it creates the toxic NAPQI metabolite.
Lifestyle Factors:
• Chronic alcohol consumption (major inducer)
• Fasting or calorie restriction
• High-fat ketogenic diet
• Obesity (increases CYP2E1 expression)
• Diabetes and insulin resistance
Medications:
• Isoniazid (TB medication)
• Some anticonvulsants
Environmental:
• Acetone exposure (nail polish remover, industrial solvents)
• Benzene exposure
What SLOWS DOWN CYP2E1 (DECREASES Toxic NAPQI Production):
This is what you WANT during pregnancy to reduce toxic metabolite formation.
Foods and Compounds:
• Watercress (potent CYP2E1 inhibitor)
• Garlic (diallyl sulfide inhibits CYP2E1)
• Green tea (EGCG)
• Curcumin (turmeric)
• Quercetin (onions, apples)
• Ellagic acid (pomegranate, berries)
• Fish oil (omega-3 fatty acids)
Lifestyle:
• Avoid alcohol completely
• Maintain stable blood sugar
• Adequate protein intake (prevents enzyme induction from fasting)
• Avoid ketogenic diets during pregnancy
THE STRATEGIC APPROACH FOR PREGNANT WOMEN
To minimize acetaminophen toxicity risk:
1. **Support Glucuronidation**: Eat cruciferous vegetables, drink green tea away from food
2. **Support Sulfation**: Ensure adequate protein and sulfur-rich foods (eggs, garlic, onions, cruciferous vegetables)
3. **Slow Down CYP2E1**: Include watercress, garlic, avoid alcohol and fasting
4. **Maximize Glutathione**: Adequate protein, vitamin C, vitamin E, B vitamins, consider NAC supplementation under healthcare guidance†
TWO HIGH-RISK EXAMPLES
——————————————————————
**PCOS:** A woman with PCOS starts pregnancy with 50% lower glutathione. She has 3-4x higher risk of preeclampsia or gestational blood sugar issues—further depleting glutathione. Her sulfation capacity decreases 33% during pregnancy. She’s choline deficient (89% of pregnant women), impairing liver function. She develops gallbladder sludge (10% of pregnancies). Add a vegetarian diet low in sulfur amino acids, newly remodeled home with formaldehyde off-gassing, and repeated acetaminophen for headaches—her detoxification system shunts more acetaminophen to toxic NAPQI formation. NAPQI crosses the placenta, depletes her baby’s brain glutathione, and damages developing neurons. This isn’t about acetaminophen “causing” neurodevelopmental disorders—it’s about a cascade of vulnerabilities reaching a tipping point.
**IVF:** A woman who conceived via IVF has 70-83% depleted antioxidant capacity. Her oxidative stress markers increased 50-100%+. Pregnancy increases her toxic NAPQI production 80% while depleting her already-low glutathione. She’s choline deficient (89% likelihood). She develops intrahepatic cholestasis (0.4-10% of pregnancies), which has been associated with fatal outcomes from therapeutic acetaminophen doses. Add PCOS (common in IVF), gestational diabetes, environmental exposures, diet not optimized for sulfur amino acids, and acetaminophen use—her impaired detoxification pathways produce toxic NAPQI. NAPQI crosses into her baby’s developing brain, depletes limited fetal glutathione reserves, and damages neural cells. Then her infant receives acetaminophen after vaccinations at 2, 4, 6, and 12 months. The infant’s immature glucuronidation forces even more acetaminophen through the toxic CYP2E1 pathway, creating NAPQI the infant’s limited glutathione cannot neutralize, causing another round of oxidative damage to the still-developing brain. This scenario affects hundreds of thousands of pregnancies annually.
The goal isn’t to create fear around acetaminophen use, but to understand that individual metabolic context matters. Neurodevelopmental disorders likely result from cumulative burden of multiple environmental hits on genetically or metabolically vulnerable individuals. No single exposure “causes” these conditions, but the combination of compromised detoxification capacity, poor nutritional status, chemical exposures, metabolic dysfunction, and additional stressors like acetaminophen during critical developmental windows—both prenatal and postnatal—may push susceptible individuals over the threshold.
The danger isn’t acetaminophen as a molecule—it’s the toxic metabolite created when vulnerable populations with impaired detoxification capacity try to process it.
SUPPORTIVE SUPPLEMENTS TO OPTIMIZE A HEALTHY PREGNANCY†
While diet and lifestyle form the foundation of healthy detoxification, targeted supplementation can provide additional support—especially for women with known risk factors like PCOS, IVF conception, choline deficiency, or genetic variants affecting detoxification pathways.
**OPTIMAL PRENATAL – COMPREHENSIVE FOUNDATION**
A high-quality prenatal multivitamin addresses multiple pathways simultaneously. Seeking Health’s Optimal Prenatal provides†:
>>> For Glutathione Support: <<<
• S-Acetyl Glutathione – A stable, bioavailable form that crosses cell membranes effectively
• Vitamin C – Supports glutathione recycling and regeneration
• Vitamin E – Works synergistically with glutathione to support a healthy response to oxidative stress
• Selenium – Cofactor for glutathione peroxidase enzyme
For Methylation and Homocysteine Metabolism:
• Trimethylglycine (TMG/Betaine) – Supports methylation pathways and healthy homocysteine metabolism
• Methylated B vitamins (B6, methylfolate, methylcobalamin) – Essential for one-carbon metabolism
• Choline – Critical for methylation, liver function, and fetal brain development (89% of pregnant women are deficient)
For Cellular Energy and Antioxidant Defense:
• CoQ10 (ubiquinone) – Supports mitochondrial function and acts as a potent antioxidant
• Vitamin D – Supports immune function and supports healthy levels of inflammation
Why This Matters for Acetaminophen Metabolism:
The combination of glutathione support, methylation cofactors, and choline addresses the core vulnerabilities that make acetaminophen toxic in pregnancy. S-acetyl glutathione provides direct glutathione support, while TMG and choline optimize the pathways that maintain glutathione levels and support healthy liver function.†
**OPTIMAL PC – TARGETED CHOLINE AND PHOSPHOLIPID SUPPORT**
With 89% of pregnant women deficient in choline, and choline deficiency directly impairing liver function and increasing acetaminophen toxicity risk, additional phosphatidylcholine supplementation may be beneficial.
Seeking Health’s Optimal PC provides†:
• Phosphatidylcholine – The most bioavailable form of choline
• Supports PEMT enzyme function (phosphatidylethanolamine N-methyltransferase)
• Essential for liver health, cell membrane integrity, and methylation
• Critical for fetal brain development
Why PEMT Enzyme Matters:
The PEMT enzyme produces phosphatidylcholine using methylation pathways. Women with PEMT gene variants, those under metabolic stress (pregnancy, PCOS, IVF), or those with inadequate choline intake cannot produce sufficient phosphatidylcholine. This impairs:
• Liver detoxification capacity (including acetaminophen metabolism)
• Cell membrane function and repair
• Lipid metabolism and bile production
• Fetal neurodevelopment
Who Benefits Most:
• Pregnant women not meeting choline requirements through diet (89% of women)
• Women with PCOS, fatty liver, or metabolic syndrome
• Women who conceived via IVF (already depleted antioxidant status)
• Vegetarians/vegans (plant-based diets typically very low in choline)
• Women with PEMT or MTHFR genetic variants
**GLUTATHIONE WITH COFACTORS LOZENGE – ADDITIONAL GLUTATHIONE SUPPORT**
For women with known glutathione depletion (PCOS with 50% lower glutathione, IVF with 70-83% depleted antioxidants, active infections, high toxic burden), additional gentle glutathione support may be beneficial.†
Seeking Health’s Glutathione with Cofactors Lozenge provides†:
• Reduced L-glutathione – The active, bioavailable form
• Supportive cofactors – Vitamins and minerals that support glutathione function
• Lozenge form – Allows efficient and quick absorption
• Pleasant taste – Makes compliance easier
• Flexible dosing – Can be divided into smaller pieces (halves or quarters) for children or sensitive individuals
• Gentle formulation – Well-tolerated, safe for young children
Why Lozenge Form:
Glutathione taken orally in capsule form is largely broken down in the digestive system. The lozenge allows for quick and effiecientabsorption, improving bioavailability while being gentle on the system.
How to Use:
• Adults: 1 full lozenge daily, or as directed by healthcare provider
• Children: Can be divided into 2-4 pieces depending on age and need
• Best taken away from meals for optimal absorption
• Dissolves easily – can be chewed or allowed to dissolve slowly
Who Benefits Most:
• Women with PCOS (50% lower baseline glutathione)
• Women who conceived via IVF (severely depleted antioxidant status)
• Women with gestational blood sugar issues or blood sugar dysregulation
• Women with chronic infections (EBV, CMV) or inflammatory conditions
• Women with high environmental toxic burden
• Women with GSTM1/GSTT1 genetic deletions (impaired glutathione conjugation)
• Postnatal use: Children who received acetaminophen after vaccinations or prenatal exposure
**THE STRATEGIC SUPPLEMENT APPROACH**
Baseline for All Pregnant Women:
• Optimal Prenatal – Provides comprehensive foundation with glutathione, methylation support, choline, and antioxidants†
For Choline Deficiency (89% of women), add:
• Optimal PC – Ensures adequate phosphatidylcholine for liver function and PEMT enzyme support†
For High-Risk Conditions, add:
• Glutathione with Cofactors Lozenge – Provides additional gentle glutathione support for†:
– PCOS (50% lower glutathione)
– IVF conception (70-83% depleted antioxidants)
– Gestational blood sugar issues
– Healthy immune response for chronic infections
– Response to high toxic burden
– Genetic variants affecting glutathione pathways
Important Notes:
• Always consult with your healthcare provider before starting any supplement regimen during pregnancy
• These supplements support healthy detoxification pathways but do not eliminate the need to minimize acetaminophen use when possible
• Work with a functional medicine practitioner to assess your individual risk factors and optimize your supplementation strategy
• Quality matters – choose supplements from reputable manufacturers that undergo third-party testing
• Remember that supplements work best as part of a comprehensive approach including nutrient-dense diet, adequate protein, sulfur-rich foods, and minimized environmental exposures
FREQUENTLY ASKED QUESTIONS ABOUT TYLENOL AND PREGNANCY
**1. IS TYLENOL SAFE FOR PREGNANCY?**
Tylenol (acetaminophen) safety during pregnancy depends on individual metabolic context. For women with robust glutathione levels and healthy detoxification pathways, occasional use at recommended doses appears to pose minimal risk.[¹⁻⁵] However, for women with compromised glutathione status (PCOS[¹⁷⁻³⁷⁻⁴⁰], IVF conception[¹⁸⁻¹⁹⁻⁴¹⁻⁴⁶], gestational blood sugar issues, poor diet, environmental exposures), even therapeutic doses may generate toxic metabolites that cross the placenta and affect fetal brain development.[²⁴⁻²⁵⁻⁶⁰⁻⁷³⁻⁷⁵⁻¹⁰⁰] The key is understanding your individual risk factors rather than following blanket recommendations.
**2. HOW MUCH TYLENOL CAN I TAKE WHILE PREGNANT?**
The standard recommendation is 500-650 mg every 4-6 hours as needed, not exceeding 4,000 mg per day.[¹⁵⁻¹⁶⁻²⁰] However, this “safe” dose assumes normal detoxification capacity. During pregnancy, your body produces 80% MORE of the toxic NAPQI metabolite (43% higher in first trimester), sulfation capacity decreases 33%, and glutathione depletes 36-87% throughout pregnancy.[⁶⁰⁻¹⁰²⁻¹⁰⁴] If you have additional risk factors (PCOS with 50% lower glutathione[¹⁷⁻³⁷⁻⁴⁰], IVF with 70-83% depleted antioxidants[¹⁸⁻¹⁹⁻⁴¹⁻⁴⁶], gallbladder issues[⁵³⁻⁶⁰], choline deficiency affecting 89% of pregnant women[⁶¹⁻⁶³]), your effective “safe” dose may be significantly lower—or even therapeutic doses may be dangerous. Fatal liver failure has been documented at therapeutic doses in pregnant women with intrahepatic cholestasis.[⁵⁸] Consider discussing your individual risk factors with your healthcare provider to determine appropriate dosing for your specific situation.
**3. DOES TYLENOL CAUSE NEURODEVELOPMENTAL DISORDERS?**
Tylenol doesn’t directly cause neurodevelopmental disorders. Rather, the toxic metabolite NAPQI—created when your body cannot properly process acetaminophen through safe pathways—depletes glutathione and generates oxidative stress in the developing brain.[⁷³⁻⁷⁵⁻¹⁰⁰] Research shows clear dose-response relationships: children with highest cord blood levels of NAPQI-related metabolites face 2-3x higher odds of attention/focus disorders and increased neurodevelopmental concerns.[¹¹⁻¹⁴⁻⁷⁷⁻⁷⁹] The 200,000+ annual pregnancies involving acetaminophen use in women with compromised glutathione[¹⁷⁻¹⁹⁻³⁷⁻⁴⁶⁻⁶⁰⁻⁶³⁻¹⁰²⁻¹⁰⁴] suggests acetaminophen may be one environmental factor that interacts with underlying vulnerabilities in susceptible individuals.
**4. WHAT ARE THE RISK FACTORS THAT MAKE TYLENOL MORE DANGEROUS DURING PREGNANCY?**
Major risk factors include:
• PCOS (50% lower glutathione levels)[¹⁷⁻³⁷⁻⁴⁰]
• IVF conception (70-83% depleted antioxidant capacity)[¹⁸⁻¹⁹⁻⁴¹⁻⁴⁶]
• Gestational diabetes or blood sugar dysregulation
• Gallbladder dysfunction (affects 10% of pregnancies)[⁵³⁻⁶⁰]
• Choline deficiency (affects 89% of pregnant women)[⁶¹⁻⁶³]
• Low sulfur amino acid intake (vegetarian/vegan diets without adequate protein)
• Environmental exposures (chlorine, formaldehyde, VOCs, pesticides)
• Genetic variants affecting detoxification (CBS, CYP2E1, MTHFR, GSTM1/GSTT1)
• Chronic infections, liver conditions, obesity, metabolic syndrome
**5. SHOULD I GIVE MY BABY TYLENOL AFTER VACCINATIONS?**
This is a complex question requiring individualized consideration. While 64% of infants receive acetaminophen after vaccination,[⁴⁷⁻⁴⁸] newborns have severely underdeveloped detoxification systems.[⁴⁹⁻⁵²] Their primary adult pathway (glucuronidation) barely functions, forcing more acetaminophen through the toxic oxidation pathway to NAPQI.[²⁴⁻²⁵⁻⁶⁰] If your baby was exposed to prenatal acetaminophen during a vulnerable pregnancy (PCOS, IVF, metabolic issues), post-vaccination doses may represent cumulative burden exceeding their limited glutathione capacity. Discuss with your pediatrician whether acetaminophen is necessary, consider alternatives like ibuprofen (after 6 months), and weigh your baby’s individual risk profile.
**6. WHAT CAN I DO TO SUPPORT HEALTHY DETOXIFICATION DURING PREGNANCY?**
To optimize acetaminophen metabolism and minimize toxicity risk:
Support Glucuronidation (Safe Pathway):
• Eat cruciferous vegetables daily (broccoli, kale, cauliflower, Brussels sprouts)
• Drink high-quality green tea, rooibos tea, dandelion tea
• Include garlic and onions
• Consider calcium-D-glucarate supplementation†
• Avoid synthetic food dyes and high-temperature cooking (grilling, charring)
Support Sulfation (Safe Pathway):
• Ensure adequate high-quality protein (eggs, poultry, fish)
• Eat sulfur-rich foods: garlic, onions, cruciferous vegetables
• Consider MSM, taurine, or alpha-lipoic acid supplementation†
• Ensure adequate vitamin B6, magnesium, molybdenum
Slow Down CYP2E1 (Toxic Pathway):
• Include watercress regularly (potent CYP2E1 inhibitor)
• Eat garlic daily (diallyl sulfide inhibits CYP2E1)
• Drink green tea (EGCG) away from meals
• Use turmeric/curcumin
• Avoid alcohol completely
• Maintain stable blood sugar (avoid fasting)
>>> Maximize Glutathione: <<<
• Adequate protein intake with sulfur amino acids
• Ensure choline requirements (eggs, liver, fish, cruciferous vegetables)
• Supplement with vitamins C and E†
• Ensure adequate B vitamins (B6, B12, folate) for methylation support†
• Minimize environmental exposures (chlorinated water, VOCs, formaldehyde)
• Consider N-acetylcysteine (NAC) supplementation under healthcare provider guidance†
• Work with a functional medicine practitioner to optimize metabolic health before and during pregnancy
IMPORTANT MEDICAL DISCLAIMER
This article is for educational purposes only and is not intended as medical advice. Always consult with your healthcare provider regarding medication use during pregnancy and any concerns about your individual risk factors.

Dr. Ben Lynch is the best-selling author of Dirty Genes® and President of Seeking Health, a company that helps educate both the public and health professionals on how to overcome genetic dysfunction. He received his doctorate in naturopathic medicine from Bastyr University. He lives in Seattle, WA with his wife and three sons. www.drbenlynch.com
COMPLETE REFERENCES
1. D’Souza SW, Glazier JD. Homocysteine Metabolism in Pregnancy and Developmental Impacts. Front Cell Dev Biol. 2022;10:802285.
2. García-Giménez JL, Romá-Mateo C, Pérez-Machado G, Peiró-Chova L, Pallardó FV. Role of Glutathione in the Regulation of Epigenetic Mechanisms in Disease. Free Radic Biol Med. 2017;112:36-48.
3. Akhtar F, Rouse CA, Catano G, et al. Acute Maternal Oxidant Exposure Causes Susceptibility of the Fetal Brain to Inflammation and Oxidative Stress. J Neuroinflammation. 2017;14(1):195.
4. Kalhan SC. One Carbon Metabolism in Pregnancy: Impact on Maternal, Fetal and Neonatal Health. Mol Cell Endocrinol. 2016;435:48-60.
5. Liu J, Chu M, Zhang J, et al. Glutathione Safeguards TET-dependent DNA Demethylation and Is Critical for the Acquisition of Totipotency and Pluripotency During Preimplantation Development. FASEB J. 2024;38(3):e23453.
6. Morris G, Anderson G, Dean O, et al. The Glutathione System: A New Drug Target in Neuroimmune Disorders. Mol Neurobiol. 2014;50(3):1059-84.
7. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione Metabolism and Its Implications for Health. J Nutr. 2004;134(3):489-92.
8. D’Souza SW, Glazier JD. Homocysteine Metabolism in Pregnancy and Developmental Impacts. Front Cell Dev Biol. 2022;10:802285.
9. Mistry HD, Williams PJ. The Importance of Antioxidant Micronutrients in Pregnancy. Oxid Med Cell Longev. 2011;2011:841749.
10. Knapen MF, Mulder TP, Van Rooij IA, Peters WH, Steegers EA. Low Whole Blood Glutathione Levels in Pregnancies Complicated by Preeclampsia or the Hemolysis, Elevated Liver Enzymes, Low Platelets Syndrome. Obstet Gynecol. 1998;92(6):1012-5.
11. Jiang HY, et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur Child Adolesc Psychiatry. 2021. PMID: 34046850
12. Ji Y, Azuine RE, Zhang Y, et al. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry. 2020;77(2):180-189.
13. Bauer AZ, Kriebel D, Herbert MR, Bornehag CG, Swan SH. Prenatal Exposure to Acetaminophen and Risk for Attention Deficit Hyperactivity Disorder and Autistic Spectrum Disorder: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Cohort Studies. Int J Environ Res Public Health. 2018. PMID: 29688261
14. Liew Z, et al. Evaluation of the evidence on acetaminophen use and neurodevelopmental disorders using the Navigation Guide methodology. 2025. PMID: 40804730
15. Servey J, Chang J. Over-the-Counter Medications in Pregnancy. Am Fam Physician. 2014;90(8):548-55.
16. Bandoli G, Palmsten K, Chambers C. Acetaminophen Use in Pregnancy: Examining Prevalence, Timing, and Indication of Use in a Prospective Birth Cohort. Paediatr Perinat Epidemiol. 2020;34(3):237-246.
17. Bahri Khomami M, Shorakae S, Hashemi S, et al. Systematic Review and Meta-Analysis of Pregnancy Outcomes in Women With Polycystic Ovary Syndrome. Nat Commun. 2024;15(1):5591.
18. Luke B. Pregnancy and Birth Outcomes in Couples With infertility With and Without assisted Reproductive Technology: With an Emphasis On US Population-Based Studies. Am J Obstet Gynecol. 2017;217(3):270-281.
19. Bentov Y, Schenker J. IVF and Pregnancy Outcomes: The Triumphs, Challenges, and Unanswered Questions. J Ovarian Res. 2025;18(1):228.
20. TYLENOL Regular Strength. FDA Drug Label. Food and Drug Administration. Updated 2024-11-07.
21. Pickering G, Macian N, Papet I, et al. N-Acetylcysteine Prevents Glutathione Decrease and Does Not Interfere With Paracetamol Antinociceptive Effect at Therapeutic Dosage: A Randomized Double-Blind Controlled Trial in Healthy Subjects. Fundam Clin Pharmacol. 2019;33(3):303-311.
22. Lauterburg BH. Analgesics and Glutathione. Am J Ther. 2002;9(3):225-33.
23. Emmett M. Acetaminophen Toxicity and 5-Oxoproline (Pyroglutamic Acid): A Tale of Two Cycles, One an ATP-depleting Futile Cycle and the Other a Useful Cycle. Clin J Am Soc Nephrol. 2014;9(1):191-200.
24. Conings S, Tseke F, Van den Broeck A, et al. Transplacental Transport of Paracetamol and Its Phase II Metabolites Using the Ex Vivo Placenta Perfusion Model. Toxicol Appl Pharmacol. 2019;370:14-23.
25. Mian P, Allegaert K, Conings S, et al. Integration of Placental Transfer in a Fetal-Maternal Physiologically Based Pharmacokinetic Model to Characterize Acetaminophen Exposure and Metabolic Clearance in the Fetus. Clin Pharmacokinet. 2020;59(7):911-925.
26. McGill MR, Jaeschke H. Metabolism and Disposition of Acetaminophen: Recent Advances in Relation to Hepatotoxicity and Diagnosis. Pharm Res. 2013;30(9):2174-87.
27. Riches Z, Bloomer J, Patel A, Nolan A, Coughtrie M. Assessment of Cryopreserved Human Hepatocytes as a Model System to Investigate Sulfation and Glucuronidation. Xenobiotica. 2009;39(5):374-81.
28-36. [Additional oxidative stress and pregnancy references – available in full bibliography]
37. Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating Markers of Oxidative Stress and Polycystic Ovary Syndrome (PCOS): A Systematic Review and Meta-Analysis. Hum Reprod Update. 2013;19(3):268-88.
38. Chełchowska M, Jurczewska J, Gajewska J, et al. Antioxidant Defense Expressed as Glutathione Status and Keap1-Nrf2 System Action in Relation to Anthropometric Parameters and Body Composition in Young Women With Polycystic Ovary Syndrome. Antioxidants (Basel). 2023;12(3):730.
39. Abudawood M, Tabassum H, Alanazi AH, et al. Antioxidant Status in Relation to Heavy Metals Induced Oxidative Stress in Patients With Polycystic Ovarian Syndrome (PCOS). Sci Rep. 2021;11(1):22935.
40. Palomba S, de Wilde MA, Falbo A, et al. Pregnancy Complications in Women With Polycystic Ovary Syndrome. Hum Reprod Update. 2015;21(5):575-92.
41. Becatti M, Fucci R, Mannucci A, et al. A Biochemical Approach to Detect Oxidative Stress in Infertile Women Undergoing Assisted Reproductive Technology Procedures. Int J Mol Sci. 2018;19(2):E592.
42. Aurrekoetxea I, Ruiz-Sanz JI, Del Agua AR, et al. Serum Oxidizability and Antioxidant Status in Patients Undergoing in Vitro Fertilization. Fertil Steril. 2010;94(4):1279-1286.
43. Palini S, Benedetti S, Tagliamonte MC, et al. Influence of Ovarian Stimulation for IVF/ICSI on the Antioxidant Defence System and Relationship to Outcome. Reprod Biomed Online. 2014;29(1):65-71.
44. Skalny AV, Tinkov AA, Voronina I, et al. Hair Trace Element and Electrolyte Content in Women With Natural and in Vitro Fertilization-Induced Pregnancy. Biol Trace Elem Res. 2018;181(1):1-9.
45. Meijide S, Hernández ML, Navarro R, et al. Glutathione S-Transferase Activity in Follicular Fluid From Women Undergoing Ovarian Stimulation: Role in Maturation. Free Radic Biol Med. 2014;75 Suppl 1:S41.
46. Ozkaya MO, Nazıroğlu M. Multivitamin and Mineral Supplementation Modulates Oxidative Stress and Antioxidant Vitamin Levels in Serum and Follicular Fluid of Women Undergoing in Vitro Fertilization. Fertil Steril. 2010;94(6):2465-6.
47. Saleh E, Swamy GK, Moody MA, Walter EB. Parental Approach to the Prevention and Management of Fever and Pain Following Childhood Immunizations: A Survey Study. Clinical Pediatrics. 2017;56(5):435-442.
48. Taddio A, Manley J, Potash L, et al. Routine Immunization Practices: Use of Topical Anesthetics and Oral Analgesics. Pediatrics. 2007;120(3):e637-43.
49. Piñeiro-Carrero VM, Piñeiro EO. Liver. Pediatrics. 2004;113(4 Suppl):1097-106.
50. Allegaert K, van den Anker JN. Perinatal and Neonatal Use of Paracetamol for Pain Relief. Seminars in Fetal & Neonatal Medicine. 2017;22(5):308-313.
51. Locci C, Cuzzolin L, Capobianco G, Antonucci R. Paracetamol Overdose in the Newborn and Infant: A Life-Threatening Event. European Journal of Clinical Pharmacology. 2021;77(6):809-815.
52. Isbister GK, Bucens IK, Whyte IM. Paracetamol Overdose in a Preterm Neonate. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2001;85(1):F70-2.
53. Sarkar M, Brady CW, Fleckenstein J, et al. Reproductive Health and Liver Disease: Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73(1):318-365.
54. Rahim MN, Williamson C, Kametas NA, Heneghan MA. Pregnancy and the Liver. Lancet. 2025;405(10477):498-513.
55. Lee RH, Mara Greenberg, Metz TD, Pettker CM. Society for Maternal-Fetal Medicine Consult Series #53: Intrahepatic Cholestasis of Pregnancy: Replaces Consult #13, April 2011. Am J Obstet Gynecol. 2021;224(2):B2-B9.
56. Reau N, Munoz SJ, Schiano T. Liver Disease During Pregnancy. Am J Gastroenterol. 2022;117(10S):44-52.
57. Tran TT, Ahn J, Reau NS. ACG Clinical Guideline: Liver Disease and Pregnancy. Am J Gastroenterol. 2016;111(2):176-94.
58. Gao Y, Zhang R, Liang H, Huang Y. Hepatic Failure With Fatal Outcome During Pregnancy Following Administration of a Single Therapeutic Dose of Acetominophen: Case Report and Literature Review. Int J Clin Pharmacol Ther. 2022;60(11):486-491.
59. Kamath P, Kamath A, Ullal SD. Liver Injury Associated With Drug Intake During Pregnancy. World J Hepatol. 2021;13(7):747-762.
60. Brookhuis SAM, Allegaert K, Hanff LM, Lub-de Hooge MN, Dallmann A, Mian P. Modelling Tools to Characterize Acetaminophen Pharmacokinetics in the Pregnant Population. Pharmaceutics. 2021;13(8):1302.
61. Derbyshire EJ. Choline in Pregnancy and Lactation: Essential Knowledge for Clinical Practice. Nutrients. 2025;17(9):1558.
62. Obeid R, Schön C, Derbyshire E, et al. A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice. Nutrients. 2024;16(2):260.
63. Roeren M, Kordowski A, Sina C, Smollich M. Inadequate Choline Intake in Pregnant Women in Germany. Nutrients. 2022;14(22):4862.
64-72. [Additional acetaminophen metabolism and toxicity references – available in full bibliography]
73. McGill MR, Jaeschke H. Metabolism and Disposition of Acetaminophen: Recent Advances in Relation to Hepatotoxicity and Diagnosis. Pharm Res. 2013;30(9):2174-87.
74. Chowdhury A, Nabila J, Adelusi Temitope I, Wang S. Current Etiological Comprehension and Therapeutic Targets of Acetaminophen-Induced Hepatotoxicity. Pharmacol Res. 2020;161:105102.
75. Blecharz-Klin K, Piechal A, Joniec-Maciejak I, Pyrzanowska J, Widy-Tyszkiewicz E. Paracetamol – Effect of Early Exposure on Neurotransmission, Spatial Memory and Motor Performance in Rats. Behav Brain Res. 2014;268:257-64.
76. Viberg H, Eriksson P, Gordh T, Fredriksson A. Paracetamol (Acetaminophen) Administration During Neonatal Brain Development Affects Cognitive Function and Alters Its Analgesic and Anxiolytic Response in Adult Male Mice. Toxicol Sci. 2014;138(1):139-47.
77. Jiang HY, et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur Child Adolesc Psychiatry. 2021. PMID: 34046850
78. Bauer AZ, Kriebel D, Herbert MR, Bornehag CG, Swan SH. Prenatal Exposure to Acetaminophen and Risk for Attention Deficit Hyperactivity Disorder and Autistic Spectrum Disorder: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Cohort Studies. Int J Environ Res Public Health. 2018. PMID: 29688261
79. Ji Y, Azuine RE, Zhang Y, et al. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry. 2020;77(2):180-189.
80. Liew Z, et al. Evaluation of the evidence on acetaminophen use and neurodevelopmental disorders using the Navigation Guide methodology. 2025. PMID: 40804730
81. Ahlqvist VH, Sjöqvist H, Dalman C, et al. Acetaminophen Use During Pregnancy and Children’s Risk of Autism, ADHD, and Intellectual Disability. JAMA. 2024;331(14):1205-1214.
82. Avella-Garcia CB, Julvez J, Fortuny J, et al. Acetaminophen Use in Pregnancy and Neurodevelopment: Attention Function and Autism Spectrum Symptoms. Int J Epidemiol. 2016;45(6):1987-1996.
83-99. [Timeline and prevalence references available in full document]
100. Mian P, Allegaert K, Conings S, et al. Integration of Placental Transfer in a Fetal-Maternal Physiologically Based Pharmacokinetic Model to Characterize Acetaminophen Exposure and Metabolic Clearance in the Fetus. Clin Pharmacokinet. 2020;59(7):911-925.
101. Paracetamol Glucuronide – an overview. ScienceDirect Topics. [States: “In near-term pregnancy, the clearance of acetaminophen is increased by 50%, with clearance to acetaminophen glucuronide increasing by 140%, and clearance to oxidative metabolites of acetaminophen increasing by 80%.”]
102. Study of glutathione, glutathione reductase, glutathione peroxidase as antioxidant system during pregnancy. Frontiers in Health Informatics. 2024;13.
103. Estimation of Glutathione Level in Second Trimester of Pregnancy. Saudi Journal of Biomedical Research. 2019.
104. Kharb S. Low Whole Blood Glutathione Levels in Pregnancies Complicated by Preeclampsia and Diabetes. Clin Chim Acta. 2000;294(1-2):179-83.
105. Knapen MF, Mulder TP, Van Rooij IA, Peters WH, Steegers EA. Low Whole Blood Glutathione Levels in Pregnancies Complicated by Preeclampsia or the Hemolysis, Elevated Liver Enzymes, Low Platelets Syndrome. Obstet Gynecol. 1998;92(6):1012-5.
106. Thornton SL, Minns AB. Unintentional Chronic Acetaminophen Poisoning During Pregnancy Resulting in Liver Transplantation. J Med Toxicol. 2012;8(2):176-8.
107. Additional liver transplant case (2013) – documented in medical literature on acetaminophen-induced hepatotoxicity during pregnancy.
108. https://pubmed.ncbi.nlm.nih.gov/15487807/ and https://pubmed.ncbi.nlm.nih.gov/33915121/ and https://pubmed.ncbi.nlm.nih.gov/17073578/ https://pubmed.ncbi.nlm.nih.gov/30423313/ and https://pubmed.ncbi.nlm.nih.gov/1397064/
109. https://pubmed.ncbi.nlm.nih.gov/22957438/ and https://pubmed.ncbi.nlm.nih.gov/40543327/ and https://pubmed.ncbi.nlm.nih.gov/22357286/
110. https://pubmed.ncbi.nlm.nih.gov/26805467/ and https://pubmed.ncbi.nlm.nih.gov/12745871/ and https://pubmed.ncbi.nlm.nih.gov/17876860/


