Timothy Schwaiger, NMD
The use of salivary cortisol in evaluating a patient’s condition can be a valuable tool for physicians. Although there is a vast quantity of research in this area, the laboratory results can be confusing when trying to choose a therapy best suited for the patient. In this article, I will discuss evidence that abnormal cortisol patterns can be a reflection of basic underlying dysfunction in physical, emotional and psychological well-being, and that treatment of hypothalamic-pituitary-adrenal (HPA) axis dysregulation requires a very individualized approach. I will start with a brief review of the HPA axis and the relationship to cortisol.
Adrenal Function and Cortisol
Adrenal function is an important subject of debate and has significant clinical relevance, especially when exploring the ramifications and intricacies of the hormone cortisol. The conventional interpretation of cortisol is often restricted to the diagnosis of hypocortisolism or hypercortisolism. The most common examples of these disorders are Addison’s disease and Cushing’s syndrome. Naturopathic medicine, in some cases, has embodied the approach of simplifying the non-conventional approach to the definition of “adrenal fatigue,” which is quite often inadequate. Therefore, I would like to expand the view to include a more detailed approach of evaluating patients with adrenal or HPA axis dysregulation. Adrenal function is complicated and involves numerous levels of functioning in the body.
The hypothalamus controls reactions to stress and regulates various body processes such as mood. This system is deeply connected to the very make-up of our physical and emotional welL
l-being. One approach to treating individuals with dysregulation of this system is to provide them with adrenal supplements or glandular products. While this can be very beneficial at times, a more elaborate understanding of the HPA axis, especially the diurnal secretion patterns of cortisol, can better assist us in evaluating and treating patients. Before we explore this idea, I will review the basic pathways and mechanism of cortisol secretion.
Cortisol Regulation
Cortisol is the primary regulator in the activity of the HPA axis by its negative feedback effects on adrenocorticotropic hormone (ACTH) and corticotropin-releasing hormone (CRH). CRH is transported to the anterior lobe of the pituitary via the portal system, where it stimulates secretion of ACTH by corticotropes. ACTH is released on a pulsatile basis, with peaks approximately every 30 minutes and a half-life of up to 15 minutes. Cortisol is then released following ACTH bursts about 30 minutes later. The negative feedback effects of cortisol are exerted at the level of both the hypothalamus and the pituitary gland. The cortisol response to ACTH bursts is quite steady; however, the bursts may fluctuate. Therefore, it is the number of secretory periods of CRH and ACTH that determines the total daily cortisol secretion. Dehydroepiandrosterone (DHEA) is also made by the adrenal glands and is thought to be an antagonist to the action of cortisol. Some studies have shown DHEA-sulfate (DHEA-s) to be decreased during times of stress.
Diurnal Changes
ACTH secretion follows a circadian rhythm, with peak cortisol blood levels attained upon awakening and a gradual decline throughout the day. There does seem to be a day-to-day variation in the pattern; however, the early morning peak with a gradual decline is “typical.” The increased cortisol secretion observed during the early morning hours and the increased secretion in response to stress result primarily from increased central nervous system (CNS) or autonomic activity, possibly also affected by vasopressin as well as cortisol. Vasopressin, or antidiuretic hormone (ADH), is secreted by the posterior pituitary and is mainly responsible for water reabsorption in the kidneys. However, vasopressin also acts as a neuropeptide/neurotransmitter, and increased secretion of this substance has been linked to depression and anxiety.
Numerous factors can have an effect on diurnal cortisol patterns. Aging, quality of sleep and time of awakening will affect this pattern. For example, in normal individuals, earlier than usual awakening is associated with increased morning peak levels of cortisol, while late awakening exhibits decreased levels. This variation is important when performing and interpreting salivary cortisol tests. In addition, conditions such as depression, anxiety, chronic fatigue syndrome (CFS), fibromyalgia, cancer and metabolic syndrome all show dysregulation of the HPA axis as exhibited by either blunted or inconsistent (atypical) diurnal cortisol patterns.
 Changes with Aging
Changes with Aging
As we grow older, there are some obvious changes that occur in the neuroendocrine system. In respect to the glucocorticoids, levels of DHEA are decreased with age. On the other hand, the diurnal rhythm of cortisol may be blunted, and a general shift to increased evening levels of cortisol has been noted in the normal, healthy elderly population. In the same manner, the ratio of cortisol to DHEA is increased with age. This occurs with other hormones as well. For example, it is not uncommon for the ratio of estrogen to testosterone to increase in elderly men. In relation to cortisol, studies suggest that frailty, depression and dementia can affect the “typical” diurnal pattern in elderly persons. One study found that elderly persons with memory deficit and/or depression demonstrate a flat or blunted diurnal pattern of cortisol. Supplementation with DHEA for these individuals has produced mixed results.
Stress
I was intrigued by the writings of Anna Dahlgren from Stockholm University when she explained the reaction of the fight-or-flight response. She states that the immediate reaction to a stressor is a catabolic activity during which time the sympathetic nervous system is activated as a response to a threat. These biochemical responses to stress take place in order to increase energy production. Anabolic activity occurs during relaxation and sleep. Consequently, if someone spends abnormally long periods in the catabolic phase without adequate anabolic activity, therein lays the imbalance and subsequent dysfunction. If these catabolic periods are temporary and intermediate, the body adapts and homeostasis is established. If the catabolic periods last longer, homeostasis is threatened.
The locus ceruleus (LC) is a very special mediating area for regulating the stress response in the body. During periods of stress, the LC will release norepinephrine, which in turn activates the HPA axis, and therefore is responsible for increasing cognitive function and motivation. Epinephrine is released by the adrenal glands in response to stress. This neurotransmitter has stimulatory effects, such as an increase in heart rate, and is well known for its key role in the fight-or-flight response. The amino acid L-glycine and neurotransmitter gamma-aminobutyric acid (GABA) have an inhibitory effect on the system.
Salivary Cortisol Patterns and Illness
Salivary cortisol assessment can aid in the evaluation of conditions such as depression, anxiety, CFS, fibromyalgia and sleep disorders. It is important, however, to realize that it is just that – a tool, and not an end-all for diagnosis. The next section will summarize some abnormal patterns seen with this testing. Some research demonstrates inconsistency in the findings, so this article does not serve as a meta-analysis of all data.
Sleep Disorders
The amount and quality of sleep one gets can have a huge effect on the HPA axis. Any time patients present with difficulty initiating and maintaining sleep (DIMS), excessive daytime somnolence (EDS) or problems related to insufficient sleep, they must be evaluated for a number of sleep disorders. Disorders of EDS include narcolepsy, idiopathic hypersomnolence and even sleep apnea, which can present with overt daytime sleepiness. In addition, disorders such as restless legs or periodic limb movements during sleep may be associated with DIMS or EDS.
Individuals with complaints of difficulty initiating and maintaining sleep exhibit dysfunctional diurnal bursts in diurnal salivary cortisol levels. A study performed on middle-aged individuals who have poor sleep quality showed increased levels of salivary cortisol upon awakening, whereas sleep-deprived individuals showed increased evening cortisol levels.
Insomnia is a subjective complaint and not a medical diagnosis. In other words, there is always an underlying cause to insomnia, whether medical or psychological. The physician must distinguish between difficulty falling asleep and staying asleep. For example, patients with CFS often report a subjective feeling of unrefreshed sleep upon awakening. However, most research shows cortisol hyposecretion in these patients. Patients with insomnia secondary to generalized anxiety are more likely to have a dysfunction of hypersecretion of cortisol. Individuals with sleep deprivation or inadequate amounts often show elevated evening cortisol.
 Depression/Anxiety
Depression/Anxiety
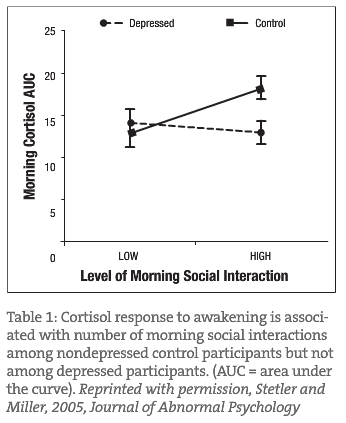
Some studies show an association of early morning cortisol hypersecretion with major depression; others suggest a “blunted” or low cortisol pattern in the early morning hours. Another study demonstrated that non-depressed individuals have increased spikes in morning cortisol levels associated with social contact in the morning hours; however, depressed people have a “blunted” response to early morning social contact. Graph 1 represents this blunted response in a group of mild to moderately depressed females. This study demonstrates the need to treat the depression and not just encourage increased social networking or increased contact with other people.
A common symptom of depression is early morning awakening. Many of my patients, prior to treatment for depression, report a “cortisol-like” surge upon awakening. In addition, if salivary cortisol is measured on early awakening it may increase this peak surge level. This surge will most likely correct itself following treatment. It may take the patient some time to adjust to this normalization. Individuals showing major depression with associated anxiety or post-traumatic stress disorder (PSTD) may demonstrate a pattern of evening cortisol hypersecretion.
Consideration in Treatment of HPA-Axis (Cortisol) Dysfunction
Relaxation Techniques
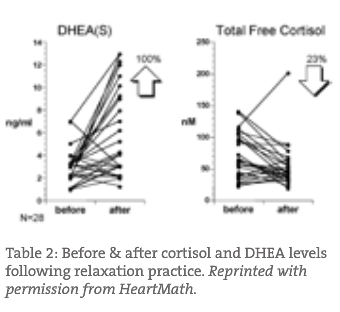
There is ample evidence that lifestyle changes can have a huge impact on the dysfunction of the HPA axis. For example, Figure 1 represents DHEA-s and cortisol levels before and after the use of a computerized display biofeedback relaxation device. There was a rise in DHEA-s levels and a reduction in free cortisol following therapy.
In a study published in Applied Psychophysiology & Biofeedback, it was reported that the use of progressive relaxation resulted in lower salivary cortisol levels and an increase in salivary immunoglobulin A (sIgA). Reduced levels of sIgA have been linked to increased upper respiratory infections in endurance runners. These relaxation techniques have also been shown to reduce conditions of high blood pressure, depression and anxiety. A study performed in Spain on preoperative patients compared the use of diazepam with listening to music on the day of surgery. It was concluded that the use of music was as effective as diazepam in reducing preoperative anxiety. Prior to this study, the same results were found in preoperative patients in Australia.
Studies have been conducted on the effects of the HPA axis and immune system using Mindfulness-Based Stress Reduction (MBSR). A one year follow-up involving patients trained in MBSR with breast and prostate cancer showed reduction in proinflammatory cytokines, reduced cortisol levels and reduction in self-reported stress levels.
Nutraceutical Interventions
Supplementation for cortisol dysfunction should be centered on trying to correct or adjust the underlying problem. Supplementation with hydrocortisone or DHEA may have a place; however, the use of these products may not be getting to the root of the patient’s condition.
The use of amino acids such as L-glycine, DL-phenylalanine (DLPA) and L-tryptophan has been a foundation in my therapy in treating depression and anxiety, as well as insomnia associated with mental disorders.
- L-glycine is a strong neurotransmitter inhibitor, acting on the locus ceruleus, giving the patient a sense of well-being and calmness without sedative effects. High doses of 3-4g three times per day may be needed to get such an effect. I almost always use L-glycine in cases of anxiety or substance abuse-related anxiety and/or withdrawal.
- L-tryptophan, in combination with melatonin, can be very effective for insomnia associated with depression and/or anxiety. A starting dose of 1-3g of L-tryptophan with 3mg of sustained-release melatonin seems to be a standard dose for my patients. L-tryptophan, of course, is a precursor to serotonin and melatonin. An increased intake of L-tryptophan results in increased brain levels of serotonin. Serotonin is found primarily in blood platelets and in the gastrointestinal tract. Other practitioners find similar results with 5-hydroxytryptophan (5-HTP).
- I use DL-phenylalanine quite often as a complement to L-tryptophan and melatonin when dealing with major depression and associated difficulty initiating or maintaining sleep. For patients suffering from chronic pain and depression, DLPA works very well. DLPA acts as an enkephalinase inhibitor, and prevents the breakdown of the brain’s natural endorphins. I use 3-9g in three divided doses depending on the situation.
- B-complex is an important adjunct therapy when treating with neurotransmitters. The vitamins are involved in numerous physiological processes.
- Phosphatidylserine (PS) has been shown to reduce levels of cortisol. It appears that this activity is somewhat dose dependent. Researchers have concluded that PS appears to lower cortisol after exercise and increase feelings of well-being while blunting muscle soreness. Similar to the results of older studies on PS, more current findings suggest that the mechanism involves a PS-induced inhibition of ACTH release by the pituitary gland. The fact that in one study ACTH did not rise in those taking PS but did increase in the placebo group substantiates the idea.
Salivary cortisol can be a valuable tool in the evaluation and treatment of many conditions. In addition, it can be a very helpful tool to evaluate progress and success of therapy. The HPA axis and diurnal patterns of cortisol are much more complex than outlined in this brief article. When using the test, it is particularly important that each patient be as consistent as possible in the collection of samples. I encourage all physicians to establish a working relationship with the laboratory of choice in obtaining the most accurate measurement possible. In theory, the patient’s success will be reflected favorably in the test results.
Case Studies
Case 1: Hypertension
A 44-year-old man was diagnosed with hypertension ten years prior to his visit to my office. During periods of stress, he stated that he had numbness in his left chest and left arm that would last up to three hours. He had been to a cardiologist, and all tests were negative for heart disease. He said that stress had always been a problem (past 20 years). He was taking 60mg of nifedipine for the past ten years, and he desired a natural approach.
Salivary cortisol testing revealed the following results:
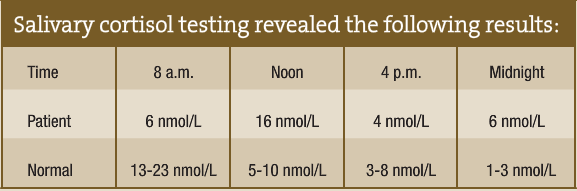
The patient was placed on 10mg DHEA each morning, 3g L-glycine three times per day and 100mg phosphatidylserine three times per day. Within three months, I was able to discontinue his blood pressure medication. Following six months of the natural protocol, he was able to eliminate all supplements without consequential blood pressure elevation. In addition to the supplements, he entered counseling and started relaxation practice.
Case 2: Anxiety and PTSD
A 48-year-old man came to my office with depression associated with PTSD, along with generalized anxiety for one year duration. He had a negative history of any organic sleep disorder; however, his total sleep time was only five to six hours. His normal bedtime was 11 p.m., and his wake time was 4 a.m. He awakened in the morning with subjective complaints of unrefreshed sleep, and excessive daytime sleepiness and fatigue. He had difficulty with periods of anxiety and worry all the time, especially in the evening.
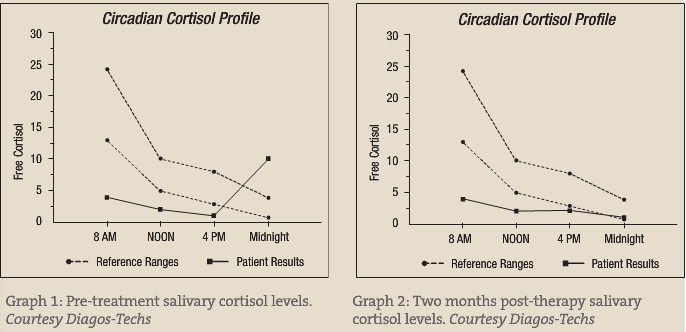
His initial salivary cortisol revealed quite elevated levels at 11 p.m. The remaining three periods basically revealed hyposecretion (see pre- and post-treatment figures). This fits the characteristic elevated cortisol seen with sleep deprivation and PTSD. The typical morning elevation in depression is not seen, which may represent more of a “blunted” effect.
Figure 2 is two months post therapy. At this time, the patient’s depression and anxiety were under control; however, he was experiencing fatigue during the day. He was still only sleeping five to six hours per night. I made the recommendation that he increase his total sleep time to seven hours, exercise daily and use full-spectrum light therapy in the morning to reinforce the circadian rhythm. My hope is to bring the diurnal pattern to a more normal range following several months of therapy. At this point, I have not repeated the cortisol test; however he reports more energy with increased sleep time and regular exercise.
 Timothy Schwaiger, NMD received his naturopathic degree from SCNM. He holds a masters degree in health services management and has more than 24 years of health-related experience. He also completed a two-year residency at SCNM in family medicine. Dr. Schwaiger has had extensive training in prolotherapy used in the treatment of pain. Among many treatment modalities, he uses nutrition and supplementation, botanical medicine, acupuncture and Chinese medicine, pharmacology, hormones, homeopathy, lifestyle modification and spinal manipulation in his practice. He is an associate professor of naturopathic medicine at SCNM.
Timothy Schwaiger, NMD received his naturopathic degree from SCNM. He holds a masters degree in health services management and has more than 24 years of health-related experience. He also completed a two-year residency at SCNM in family medicine. Dr. Schwaiger has had extensive training in prolotherapy used in the treatment of pain. Among many treatment modalities, he uses nutrition and supplementation, botanical medicine, acupuncture and Chinese medicine, pharmacology, hormones, homeopathy, lifestyle modification and spinal manipulation in his practice. He is an associate professor of naturopathic medicine at SCNM.
References
Rhoades R and Tanner G: Medical Physiology, Philadelphia, 1995, Lippincott Williams & Wilkins.
Isselbacher et al: Harrison’s Principles of Internal Medicine, New York, 1994, McGraw Hill, p.1955.
Kalimi M et al: Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA), Mol Cell Biochem 131:99-104, 1994.
Edwards S et al: Association between time of awakening and diurnal cortisol secretory activity, Psychoneuroendocrinology Aug; 26(6):613-22, 2001.
Frank E and Landgraf R: The vasopressin system, from antidiuresis to psychopathology, Eur J Pharmacol Apr 7;583 (2-3):226-42, 2008.
Dockray S et al: The cortisol awakening response in relation to objective and subjective measures of waking in the morning, Psychoneuroendocrinology Jan; 33 (1):77-82, 2008.
Ahn RS et al: Salivary cortisol and DHEA levels in the Korean population: age-related differences, diurnal rhythm, and correlations with serum levels, Yonsei Med J Jun 30; 48(3):379-88, 2007.
Dahlgren A et al: Overtime work and its effects on sleep, sleepiness, cortisol and blood pressure in an experimental field study, Scandinavian Journal of Work, Environment and Health 32(4):318-27, 2006.
Lasikiewicz N et al: Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: associations with sleep quality and metabolic parameters, Psychoneuroendocrinology Feb; 33 (2):143-51, 2008.
Buckley TM and Schatzberg AF: On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorder, Journal of Clinical Endocrinology & Metabolism 90(5):3106-3114, 2005.
Mannie Zola N et al: Increased waking salivary cortisol levels in young people at familial risk of depression, American Journal of Psychiatry 164(4):617-621, April 2007.
Duval et al: Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: dopaminergic, noradrenergic and thyroid correlates, Psychoneuroendocrinology Aug;31(7):876-88, 2006.
Hugo FN et al: Association of chronic stress, depression symptoms and cortisol with low saliva flow in a sample of south-Brazilians aged 50 years and older, Gerodontology Mar; 25(1):18-25, 2008.
Stetler C and Miller GE: Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts, J Abnorm Psychol Nov; 114 (4):697-705, 2005.
Young EA and Breslau N: Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study, Biological Psychiatry Aug 1;56(3):205-9, 2004.
McCraty R et al: The impact of a new emotional self-management program on stress, emotions, heart rate variability, DHEA and cortisol, Integrative Physiological and Behavioral Science 33(2):151-170, 1998.
Nieman DC et al: Relationship between salivary IgA secretion and upper respiratory tract infection following a 160-km race, J Sports Med Phys Fitness Mar;46(1):158-62, 2006.
Berbel P et al: Music vs. diazepam to reduce preoperative anxiety: a randomized controlled clinical trial, Rev Esp Anestesiol Reanim Jun-Jul; 54(6):355-8, 2007.
Cooke M et al: The effect of music on preoperative anxiety in day surgery, Adv Nurs Oct; 52(1):47-55, 2005.
Carlson LE et al: One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients, Brain Behav Immun Nov; 21(8):1038-49, 2007.
Hellhammer J et al: Effects of soy lecithin phosphatidic acid and phosphatidylserine complex (PAS) on the endocrine and psychological responses to mental stress, Stress Jun;7(2):119-26, 2004.
Fahey TD and Pearl M: Hormonal effects of phosphatidylserine during 2 weeks of intense training, abstract submitted to national meeting of the American College of Sports Medicine, June 1998.
Pawlow LA and Jones GE: The Impact of Abbreviated Progressive Muscle Relaxation on Salivary Cortisol and Salivary Immunoglobulin A (sIgA), Appl Psychophysiol Biofeedback December;30(4):375-87, 2005.


