The Wound That Does Not Heal
Kelly Marie Fitzpatrick, ND, BSN, MPS
Acute inflammation by definition is a finite self-limiting presentation.1,2 The phases of acute inflammatory healing include the inflammatory phase, proliferative phase, and maturation phase,1,2 which lead to the repair of an inflammatory condition. All chronic inflammation begins as acute inflammation but does not follow the well-orchestrated process of acute inflammation.3 Key cellular components of acute inflammation and angiogenesis diversify and redifferentiate as the chronicity of inflammation in the environment ensues. Observations suggest that chronic inflammation is involved in tumor initiation, promotion, and progression.4-6
In 1863, Rudolf Virchow recognized leukocytes in neoplastic tissues and suggested that the “lymphoreticular infiltrate” at sites of chronic inflammation had implications in carcinogenesis.4-9 These chronic infections and inflammatory presentations contribute to 15% to 25% of all cancer conditions worldwide. As summarized in Table 1, examples of chronic inflammatory conditions that are associated with cancer risks include the following: Schistosoma hematobium with bladder cancer, Helicobacter pylori with gastric adenocarcinoma, hepatitis virus with cirrhosis and hepatocellular carcinoma, asbestos-induced inflammation with bronchogenic carcinoma or mesothelioma and cryptogenic inflammatory conditions (ie, prostatitis for prostate cancer), and human papilloma virus with cervical cancer.4-6,9-15 The oncology profession recognizes 15 oncogenic human papilloma virus types that contribute to cervical dysplasia and potential carcinoma.16
In 1986, Harold Dvorak suggested that “tumors are wounds that do not heal.”7(p) His observations were that the mechanisms of wound healing and the formation of tumor stroma had similar connective tissue components, including fibroblasts, blood and lymphatic vessels, inflammatory cells, and extracellular matrix.7 In contrast to healing wounds, chronicity of the inflammatory phase results in uncontrolled cell proliferation, invasion, and metastasis (Figure 1).7 Most of these susceptible cells that transform when exposed to metabolic stress are of epithelial cell origin (carcinomas).17 In patients with cancer, wound healing may be compromised by several factors, including age, nutrition, comorbidities, and lifestyle, as well as therapeutic modalities, including chemotherapy, radiation therapy, corticosteroid use, and surgery.18
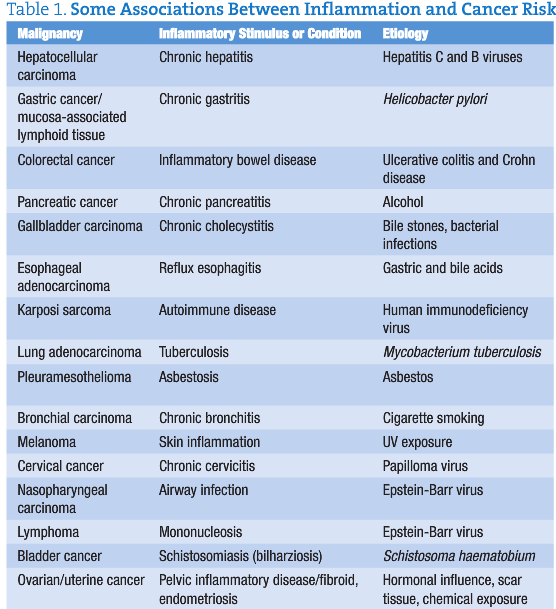
Figure 1. Cellular parallels between a tumor and a skin wound. Both types of tissue are characterized by the presence of a fibrin clot, inflammatory cells (neutrophils, macrophages, mast cells, and lymphocytes), newly formed blood vessels, and a large number of fibroblasts and myofibroblasts. In addition, migrating and proliferating keratinocytes are present in the wound and in the cancer tissue. The main difference between tumors and wounds is the invasive growth of the transformed keratinocytes.7
Pathogen-Associated Inflammation
Gastric cancer, the second most common cause of cancer mortality worldwide, is associated with the pathogen Helicobacter pylori. There is a generalized increased risk for the progression of cancer after exposure to inflammatory conditions of esophagitis, gastritis, colitis, pancreatitis, and hepatitis. Pathogen-induced chronic inflammation in the intestine promotes apoptosis of normal cells that can lead to proliferation of aberrant tissue, epigenetic modification, DNA damage, and cell cycle arrest.5,6,13 Leukocytic infiltration characterizes the pathogenic progression of chronic inflammation. Within the lumen of the gut, epithelial cells are responsible for generating proinflammatory cytokines that recruit and activate innate and adaptive immune responses.13 Other inflammatory cells are attracted to the site by mediators released by mast cells; monocytes differentiate into macrophages and are activated in response to local inflammatory chemokines and cytokines.5 Apparently, the cytokine profile at the site of inflammation determines the outcome and progression toward carcinogenesis. In H pylori, the nature of cytokines of acute inflammation controlling epithelial transformation, angiogenesis, cellular proliferation, and remodeling favors an increase in proinflammatory cytokines. Often, eradication of the pathogen and the use of anti-inflammatory agents (ie, cyclooxygenase 2 inhibitors) prevent progression of these gastric cancers.8,9
Paradoxical Mechanisms
At this juncture, we are challenged by a quandary. Does a tumor facilitate its existence through the manipulation of the immune system and the promotion of chronic inflammatory mechanisms, or does a chronic inflammatory condition proactively recruit and transform stem cells into tumor cells? Both questions have relevance in the argument of tumorigenesis. The interplay and paradoxical patterns of innate and adaptive cellular communication promote a proinflammatory and protumorigenic environment that allows tumors to grow and escape immune surveillance.9 Immune system representatives of the inflammatory milieu in acute, chronic, and carcinogenic tissue exhibit paradoxical mechanisms of suppression and enhancement of the inflammatory state and the tumor potentiality. The innate immune system, composed of myeloid lineage cells, and the adaptive immune system, composed of T cells and B cells, function rationally and irrationally in response to an orchestration of chaos, reflecting a disruption in the immune response to potentially lethal cells and a collaboration with these mutant cells to allow a deterioration of the homeostatic principles of the host (Table 2). Monocytes, platelets, macrophages, mast cells, dendritic cells, neutrophils, basophils, and natural killer cells preside peripherally and within the inflammatory tissue environment, responding to the secretions of pleiotropic cytokines, chemokines, proteases, metalloproteinases, reactive oxygen and nitrogen species, and other bioactive mediators10-12 (Figure 2).
 How fascinating it is to witness the intricacies of molecular mechanisms during acute and chronic inflammation. At determined points, the inflammatory pathways benefit the host through the repair and remodeling of the extracellular matrix and vascular system, removal of bacterial proliferation through oxidative bursts and phagocytic engulfing, and appropriate surveillance of renegade cellular division. On the other hand, these same custodians of tissue trauma, pathogenic invasion, impaired cellular growth, angiogenesis, and oxidative damage will become traitors to the well-being of the host after reaching critical mass in the chronic state of disease. During chronic inflammation, the innate immune cells have a threshold capacity to maintain equilibrium and surveillance. At a certain point, the regulation of homeostasis and disease pathogenesis responds more dramatically to the mechanisms of pathogenesis and tumorigenesis. Proinflammatory cytokines and the sustained generation of reactive oxygen species and reactive nitrogen species contribute to neoplastic cell proliferation and survival of damaged epithelial tissue. Neoplastic tissue can escape the immune system surveillance directly and indirectly through alterations in the regulatory T lymphocytes.10 In the region of neoplastic development in association with chronic inflammation, the collaboration between the innate and adaptive immune systems can favor the development of cancer progression.9-12
How fascinating it is to witness the intricacies of molecular mechanisms during acute and chronic inflammation. At determined points, the inflammatory pathways benefit the host through the repair and remodeling of the extracellular matrix and vascular system, removal of bacterial proliferation through oxidative bursts and phagocytic engulfing, and appropriate surveillance of renegade cellular division. On the other hand, these same custodians of tissue trauma, pathogenic invasion, impaired cellular growth, angiogenesis, and oxidative damage will become traitors to the well-being of the host after reaching critical mass in the chronic state of disease. During chronic inflammation, the innate immune cells have a threshold capacity to maintain equilibrium and surveillance. At a certain point, the regulation of homeostasis and disease pathogenesis responds more dramatically to the mechanisms of pathogenesis and tumorigenesis. Proinflammatory cytokines and the sustained generation of reactive oxygen species and reactive nitrogen species contribute to neoplastic cell proliferation and survival of damaged epithelial tissue. Neoplastic tissue can escape the immune system surveillance directly and indirectly through alterations in the regulatory T lymphocytes.10 In the region of neoplastic development in association with chronic inflammation, the collaboration between the innate and adaptive immune systems can favor the development of cancer progression.9-12
Macrophages
As part of the innate immune system, macrophages have a key role in the first line of defense, cellular cleanup and bacterial phagocytosis during acute inflammation and wound repair.19,20 We observe the paradoxical relationship between the healing role of macrophages (organizing immune defenses and tissue repair processes) and the detrimental procarcinoma role of macrophages (producing molecular cytokines, growth factors and mediators of inflammation, matrix remodeling, tumor cell invasion, intravasation, angiogenesis, and seeding at distant sites).15,19,21,22 Prognosis is often associated with the presence of tumor-associated macrophages (TAMs) in the stroma environment.
Proinflammatory cytokine expression by the TAMs during H pylori infection has been noted as influential to tissue transformation, tumor cell proliferation, angiogenesis, invasion, and metastasis.4,5,22 High TAM densities in breast, prostate, ovarian, and cervical cancers are associated with poor prognosis.20
Bone Marrow–Derived Cell Recruitment
An important consequence of inflammatory activity in epithelial cell cancer is the recruitment of bone marrow–derived cells (BMDCs).9 Chronic inflammation attracts BMDCs. Locally, the BMDCs have been shown to populate an area of inflammation, contribute to abnormal tissue development, and distribute themselves into the epithelial stem cell niche.5,9 These BMDCs exhibit remarkable plasticity and differentiate into cells of varied lineage. The differentiation of the BMDCs is actually governed by chemotaxis and the tissue microenvironment. There is research and speculation regarding the translocation of cells to the site of chronic inflammation due to the release of chemoattractants, whereby the BMDCs fuse and differentiate into the developing vasculature of the tumor and its microenvironment.9
Oxidative and Nitrosative Stress
The release of reactive oxygen and nitrogen species into the cellular environment by neutrophils and macrophages at the site of inflammation has a role in bacterial killing and recruitment of molecular players. Reactive species are produced in the mitochondria, cytochrome P-450, and peroxisomes in all cells. Under normal circumstances, reactive oxygen and nitrogen species are important in intracellular signaling, gene expression, and physiological function. A balance of the benefits of these reactive species and the consequences of prolonged generation of these radical intermediates characterizes the mechanisms by which epigenetic damage occurs and the risk of cancer development increases.23,24 Oxidative stress refers to a favored increase of reactive oxygen intermediates (such as hydroxyl and superoxide radicals) and hydrogen peroxide and singlet oxygen. Nitrosative stress refers to a favored increase of reactive nitrogen intermediates such as nitric oxide, peroxynitrite, and S-nitrosothiols. Metabolic stress refers to the reactive intermediate role in cellular and extracellular metabolic events. The reactions of the reactive oxygen intermediate and reactive nitrogen intermediate species with proteins, carbohydrates, and lipids may disrupt the intracellular and extracellular arena, resulting in cell death, regeneration, proliferation, and tumor formation.23
Naturopathic Considerations and Approaches
As traditional practitioners, naturopathic physicians treat the mechanisms of inflammation at every juncture of patient care. Key among the interventions of reducing the inflammatory mechanisms are focuses on the role of nutrition and on the removal of physical and emotional stressors. Only 5% to 10% of cancers are associated with genetic history (5%) or family history (5%), while obesity and dietary habits account for 35%, environmental influences for 25%, and lifestyle factors for 30%.25 According to Meng and Riordan,26 cancer treatments should comprise 3 approaches: (1) removal of the cause of persistent inflammation or unhealed wounds, (2) provision of enough repair cells to the site of inflammation and malignant neoplasm, and (3) delivery of sufficient gene repair factors and substrates necessary for healing the inflammation or wound at the site of malignancy. Substrate deficiency can lead to incomplete healing at sites of inflammation; thus, adequate nutrient loading may be necessary to provide whole cellular nutrition.26
The following is an herbal formula that I use as part of an integrated medical approach for patients who present with gastrointestinal disturbances from ulcerations, esophagitis, inflammatory bowel disease, Crohn disease, and gastric cancer: Oplopanax horridus (2.5), Mahonia aquifolium (2), Achillea millefolium (1.5), Crataegus oxycantha (1), and Caldenfula officinalis (1). The prescription is marked 30 drops 4 times daily; modify with symptom picture.
Botanicals
Oplopanax horridus (devil’s club) is native to the northwest has been used medicinally by indigenous tribes and Western herbalists for various ailments. In vitro studies using the extract of the root bark of O horridus showed that this botanical was able to inhibit cell proliferation of human leukemia, non–small cell lung cancer cell lines, and breast cancer cell lines. Another in vitro study using O horridus fractions on human colorectal cancer cells exhibited antiproliferative activity on this cell line also. The most active fractions in these studies were 70% and 100% ethanol extractions.18,19
Mahonia (Berberis) aquifolium (Oregon grape) root extract was found to inhibit the release of proinflammatory cytokines, inhibit the chemotaxis of T cells toward inflammatory regions, decrease the single-strand cleavage of DNA, quench reactive oxygen and reactive nitrogen species, and increase cell viability.20,27 Berberine also exhibited antimutagenic activity against induced mutagenicity with chemical agents.28 Berberine is biologically active against gram-positive and gram-negative bacteria, fungi, protozoa, trypanoxomes, and plasmodia.29-31
Achillea millefolium (yarrow) has been used as an anti-inflammatory, antispasmodic, diaphoretic bitter digestive tonic internally and has been applied externally for a wide range of conditions. This herb inhibits matrix metalloproteinases, strengthens the antioxidant defense, protects the gastric mucosa against acute and chronic lesions, and demonstrates cytotoxic effects that are selective against malignant and healthy cell lines.32
Crateagus oxycantha (hawthorne) contains flavonoids and has been used therapeutically for cardiac and arterial trophic tonifying. A mouse study33 looked at the antiulcerative properties of C oxycantha and found it to be comparable to ranitidine as an antiulcer agent.
Calendula officinalis (marigold) is mostly considered for skin disruptions and as a lymphagogue for pelvic and breast tissue. It is also an herb that is used to reduce inflammation of the gastric tract. When given to animals after induced thermal burns, the experimental animals showed significant improvement in healing.34 Calendula officinalis was also shown to possess cytotoxic and tumor-reducing potential.34
Conclusions
The mechanisms associated with infections of epithelial tissue that promote inflammation can reach a critical mass in chronic disease and have been shown to have strong associations with tumor promotion. Not all infections lead to this outcome. Chronic disease is a process that evolves over time, with the imbalance in the immune system surveillance and the potential oxidative and nitrogen stress on cellular health resulting after prolonged exposure to inflammatory mechanisms. The fact that anti-inflammatory treatment has been shown to reduce tumor progression is important in the application of therapies to reduce these phenomena. Notably, the inflammatory mechanisms that are observed are both part of the healing process and are crucial in the promotion of more serious pathologic conditions. As naturopathic physicians, we are instrumental in applying our principles of medicine and in making a difference in our patients’ lives by treating the underlying causes that are associated with the induction of acute and chronic inflammation that results in potential tumor progression.
 Kelly Marie Fitzpatrick, ND, BSN, MPS is a graduate of Bastyr University. She has been a clinical practitioner for more than 20 years. With 80%-90% of her client base being obese, she is proactive in working with them to reduce their weight and the inflammatory mechanisms that influence their well-being and contribute to the potential cancerous and chronic disease risks. She practices in Eugene and Portland, Ore.
Kelly Marie Fitzpatrick, ND, BSN, MPS is a graduate of Bastyr University. She has been a clinical practitioner for more than 20 years. With 80%-90% of her client base being obese, she is proactive in working with them to reduce their weight and the inflammatory mechanisms that influence their well-being and contribute to the potential cancerous and chronic disease risks. She practices in Eugene and Portland, Ore.
References
- Hess CT, Kirsner RS. Orchestrating wound healing: assessing and preparing the wound bed. Adv Skin Wound Care. 2003;16(5):246-256.
- Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20(1):39-53.
- Boderick N. Nurse Pract. 2009;34(10):17-22.
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545.
- Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda). 2008;23:350-358.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380.
- Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628-638.
- Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8(20):3267-3273.
- Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8(13):2005-2013.
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37.
- Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3-10.
- Burstein E, Fearon ER. Colitis and cancer: a tale of inflammatory cells and their cytokines. J Clin Invest. 2008;118(2):464-467.
- Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13(suppl 1):13-18.
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:5:1175-1183.
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263-266.
- Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97(14):1066-1071.
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:2:216-226.
- Li XL Sun S, Du GJ, et al. Effects of Oplopanax horridus on human colorectal cancer cells. Anticancer Res. 2010;30:295-302.
- Sun S, et al. Phytother Res. 2010.
- Donsky H, Clarke D. Reliéva, a Mahonia aquifolium extract for the treatment of adult patients with atopic dermatitis. Am J Ther. 2007;14:442-446.
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514-525.
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211-217.
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Int J Cancer. 2007;121:2381-2386.
- Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(spec No. 1):S23-S31.
- Alschuler LN, Gazella KA. The Definitive Guide to Cancer. 3rd ed. New York, NY: Celestial Arts; 2010.
- Meng X, Riordan NH. Cancer is a functional repair tissue. Med Hypotheses. 2006;66:486-490.
- Rackova L, Oblozinsky M, Kostalova D, Kettmann V, Bezakova L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J Inflamm (Lond). 2007;4:e15.
- Cernáková M, Kost’álová D, Kettmann V, Plodová M, Tóth J, Drímal J. Potential antimutagenic activity of berberine, a constituent of Mahonia aquifolium. BMC Complement Altern Med. 2002;2:e2.
- Cernáková M, Kostálová D. Antimicrobial activity of berberine: a constituent of Mahonia aquifolium. Folia Microbiol (Praha). 2002;47(4):375-378.
- Slobodníková L, Kost’álová D, Labudová D, Kotulová D, Kettmann V. Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids. Phytother Res. 2004;18(8):674-676.
- Volleková A, Kost’álová D, Kettmann V, Tóth J. Antifungal activity of Mahonia aquifolium extract and its major protoberberine alkaloids. Phytother Res. 2003;17:834-837.
- Nemeth E, Bernath J. Biological activities of yarrow species (Achillea spp.). Curr Pharm Des. 2008;14:3151-3167.
- Tadić VM, Dobrić S, Marković GM, et al. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J Agric Food Chem. 2008;56:7700-7709.
- Chandran PK, Kuttan R. Effect of Calendula officinalis flower extract on acute phase proteins, antioxidant defense mechanism and granuloma formation during thermal burns. J Clin Biochem Nutr. 2008;43:58-64.