Glutamine: A Solution for Chemotherapy-Induced Peripheral Neuropathy?
Malea MacOdrum, NCNM
Loch chandler, ND, Msom, lac
Student Scholarship – Honorable Mention Case Study
Peripheral neuropathy is a serious and often treatment-limiting side effect of several common cancer chemotherapeutics. Aside from impacting activities of daily living (ADLs), severe neuropathy may result in postponing potentially life-extending treatment, which ultimately may lead to increased morbidity and mortality. The use of glutamine has been shown to be successful in mitigating neuropathy. This paper will explore current research examining the efficacy of oral glutamine, and offer recommendations for the clinical use of glutamine in patients with chemotherapy-induced peripheral neuropathy (CIPN).
Chemotherapy & Peripheral Neuropathy
Of the myriad chemotherapeutics that may cause peripheral neuropathy, this symptom is particularly prominent with oxaliplatin (most often used in conjunction with leucovorin and 5-fluorouracil for the treatment of colorectal cancer) and paclitaxel (commonly used in advanced breast and ovarian cancers). Oxaliplatin may produce acute (during or immediately after treatment) or cumulative (with prolonged treatment) neuropathy. In the acute setting, neuropathy is thought to be due to the interaction of oxaliplatin with voltage-gated sodium channels.1 However, over multiple courses of oxaliplatin, a progressive neuropathy commonly develops as platinum compounds accumulate in the dorsal root ganglion, causing atrophy and mitochondrial dysfunction in peripheral nerves.2 Unlike the acute neuropathy, this form of neuropathy is largely irreversible. Paclitaxel is thought to affect predominantly small sensory fibers, as well as motor and large sensory fibers at higher doses, by disturbing microtubule dynamics, which in turn leads to axonal degeneration.3
Glutamine & Chemotherapy-Induced Peripheral Neuropathy
Despite varying mechanisms of action, glutamine has been shown efficacious in the treatment and prevention of both acute and chronic CIPN.
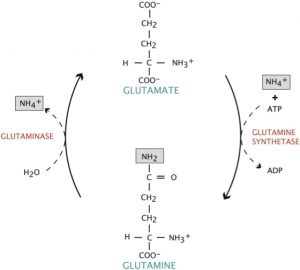
Glutamine is the most abundant free amino acid in the human body and is found naturally in most meat and dairy products, as well as cabbage, beets, beans, and spinach. It plays a critical role in a variety of physiological functions, particularly as a shuttle for nitrogen groups between tissues. It can act as a form of fuel in place of glucose, particularly for enterocytes lining the gut, and is primarily synthesized and stored in skeletal muscle. As a non-essential amino acid, glutamine may be synthesized by glutamine synthetase from glutamate and ammonium (Figure 1). However, during times of severe stress (such as those elicited with chemotherapy), glutamine becomes a conditionally-essential amino acid. Glutamine is one of the only amino acids capable of crossing the blood-brain barrier, where it is taken up by neurons and converted to the excitatory neurotransmitter, glutamate, by the enzyme glutaminase. For this reason, glutamine supplementation is often not recommended for individuals with brain cancers, namely gliomas, as carcinogenic brain tumor cells produce glutamate. The increase in local glutamate concentration then induces excitotoxic cell death in surrounding neurons, which creates space for the expanding tumor.4
Figure 1. Conversion of Glutamate to Glutamine
Literature Review
To date, only 4 clinical studies have examined the efficacy of glutamine in the treatment of chemotherapy-induced peripheral neuropathy (Table 1). Of these 4 studies, 3 examined glutamine in conjunction with paclitaxel (Vahdat,5 Stubblefield,6 Loven7) and 1 with oxaliplatin (Wang8). In addition, 1 of the studies chose to examine glutamate in lieu of glutamine (Loven). All studies had small sample sizes (Wang was the largest, with 86 study participants) and only 2 were randomized (Wang and Loven). All 4 studies failed to show statistically significant changes in nerve conduction studies, but 3 of the 4 did show significant improvements in activities of daily living, as well as improved signs and symptoms of peripheral neuropathy (Vahdat,5 Stubblefield,6 Wang8). The only study that did not yield significant improvements in subjective measures was Loven’s study, which examined glutamate in lieu of glutamine.7
Table 1. Summary of Glutamine Studies
| Study | Design | Chemo and Cancer | Glutamine Dose/Duration | Results/End Measures |
| Vahdat (2001)5 | Not randomized; not placebo-controlledTest (n=12) Control (n=33) | PaclitaxelBreast(stage IV) | 10 g PO TID x 4 days, 24 h after completion of paclitaxel | Nerve Conduction: No statistically significant change in amplitude and conductive velocity of motor and sensory nerves from baselineReflexes: Not statistically significantToe numbness: 42% test vs 82% control (p=0.009)Weakness: 25% test vs 58% control (p=0.04)ADL Interference: 27% test vs 86% control (p=0.001)
Gait Disturbance: 45% test vs 85% control (p=0.016) |
| Stubblefield (2005)6 | Not randomized; not blindedTest (n=17)Control (n=29) | PaclitaxelBreast(stage IV)
|
10 g PO TID x 4 days, 24 h after completion of paclitaxel | Nerve Conduction: 14% tibial and 9% perineal nerve difference (not statistically significant)Weakness: 0% test vs 29% control (p=0.02)¯Vibratory Sensation: 67% test vs 96% control (p=0.04)¯ Reflexes: 33% test vs 46% control (p=0.28)Symptoms: Test group had ¯ toe numbness (p=0.004), finger numbness (p=0.06) |
| Wang (2007)8 | RandomizedTest (n=42) Control (n=44) | OxaliplatinColorectal (stage IV) | 15 g PO BID x 7 days q 2 wk, starting on the day of oxaliplatin infusion | Nerve Conduction: 21.4% test abN vs 25.0% control abN (not statistically significant; p=0.68)ADL Interference: 16.7% test vs 40.9% control (p=0.02)High-Grade PN after 6 cycles: 11.9% test vs 31.8% control (p=0.04) |
| Loven (2009)7 | Randomized; double-blind; placebo-controlledTest (n=23)Control (n=20) | PaclitaxelAdvanced epithelial ovarian | 500 mg glutamate PO TID throughout 6 cycles of chemo until 3 wk after last treatment | Nerve Conduction: 30.4% test abN vs 30% control abN (not statistically significant)Weakness: 0% test vs 22.2% control (p=0.187; not statistically significant)Tingling: 52.2% test vs 50.0% control (p=0.147; not statistically significant)Numbness: 34.8% test vs 50.0% control (p=0.109; not statistically significant)Pain: 13.0% test vs 50.0% control (p=0.011) |
(ADL = activities of daily living; PN = peripheral neuropathy; abN = abnormal)
Discussion
While none of the studies showed significant changes in objectively measured nerve conduction, researchers are conflicted as to the clinical usefulness of such nerve studies in determining a therapeutic course.8 It is, however, of academic interest why nerve conduction studies have shown nerve degeneration in individuals treated with either oxaliplatin or paclitaxel in conjunction with glutamine supplementation, in the absence of advanced clinical symptoms of peripheral neuropathy. It is probable that the studies were simply not large enough to yield statistically significant results. More likely, glutamine exerts its neuroprotective effects in a way not captured by traditional nerve conduction studies.
The only study not to yield significant improvements in quality-of-life measures and the subjective experience of neuropathy was that by Loven.7 Interestingly, this was also the only study examining the efficacy of glutamate instead of glutamine. As discussed above, glutamine and glutamate are readily transferred in vivo from one to another. As the enzyme required to convert glutamate to glutamine, glutamine synthetase, is intracellular, glutamate must first cross the cellular membrane before conversion is possible. However, glutamate does not readily traverse the cell membrane, due to its negative charge.7 Entry into the cell requires a specialized amino acid transporter, present in only small amounts except the central nervous system, where glutamate is most biologically active. Loven et al theorized that paclitaxel might further downregulate or destroy these transporters as part of its neurotoxic effect.7 Glutamate, therefore, is an ineffective replacement for glutamine in the treatment of peripheral neuropathy secondary to chemotherapy.
Many medical doctors and several naturopathic doctors have suggested that glutamine may be deleterious to cancer patients. The rationale behind this concern is that cancer cells readily utilize glutamine as a fuel source in addition to glucose, as it undergoes aerobic glycolysis, as described by the Warburg effect. In addition, in cancer cells, glutamine acts as an intermediary for a number of amino acids necessary for cellular growth and proliferation. Further supporting this argument is the ability of cancer cells to more efficiently transport glutamine into mitochondria for metabolic activity, as well as the greater activity of glutaminase in cancer cell mitochondria.9 This is seen clinically as progressive decreases in a patient’s intrinsic glutamine stores that parallel increases in tumor size and activity.10 Clinically, in vitro studies of HeLa cell lines (immortal cervical cancer cells derived from patient Henrietta Lacks) have shown maximal tumor cell proliferation with glutamine concentrations of at least 1 mmol/L.11 However, in vivo studies have failed to show a connection between glutamine supplementation and increased tumor activity.12 One study examining the effects of oral glutamine showed a 40% decrease in tumor growth and a 30% increase in natural killer (NK) cell activity in rats treated with glutamine for 3 weeks.13 Further, all of the effect was reversed when also supplemented with ketamine, a potent NK cell suppressor, indicating that tumor growth was slowed by increases in NK cell counts stimulated by glutamine supplementation. While some concern regarding supplementation in brain cancers is warranted, as discussed previously, glutamine supplementation at clinically therapeutic dosages has not been shown to promote tumor progression in vivo and may actually stimulate host immune function to decrease tumor growth.
Aside from maintaining quality of life, the main reason the medical community is concerned with chemotherapy side effects is that they may potentially be dose-limiting. This may result in decreased doses, a reduction in treatment frequency, or complete cessation of a chemotherapeutic regimen. The result of such scenarios may be decreased lifespan, in the order of months to years. As peripheral neuropathy is often cited as a dose-limiting toxicity of both oxaliplatin and paclitaxel, proactively preventing or slowing the progression of neurotoxicity should yield increased survivorship. Rat models have shown that significantly higher doses of chemotherapeutics may be administered when combined with glutamine supplementation,3 and physicians consulted for this article have found similar results with their human patients. The only study to track overall effects of glutamine supplementation on chemotherapy dose reductions was the Wang study, which showed that the treatment group required fewer oxaliplatin dose reductions (7.1% vs 27.3%; p=0.02).8 However, despite greater compliance to the established oxaliplatin regimen, this did not confer a lengthening of life. In fact, the control group had a longer median survival, though this was not statistically significant (17.3 months of treatment vs 18.6 months for controls; p=0.90).8
Conclusion
Even without significant changes in overall mortality or nerve conduction studies, the positive influence of glutamine supplementation on the subjective experiences of patients cannot be dismissed. Maintaining a baseline quality of life is crucial in preserving patients’ sense of autonomy and wellness of spirit, mind, and body. On this front, glutamine has shown to be an extraordinarily useful tool in slowing the progression of peripheral neuropathy and preserving activities of daily living. While more research is needed, particularly tracking overall survivorship of individuals treated concurrently with glutamine and chemotherapy, existing studies do clearly demonstrate both the safety and efficacy of glutamine supplementation in individuals undergoing oxaliplatin and paclitaxel treatment. Based on existing research, 30 g daily in divided doses, beginning on the day of chemotherapy treatment and continuing for at least 4 days, should be considered for any patient undergoing treatment with oxaliplatin or paclitaxel.
 Malea MacOdrum is a 6th year student in naturopathic and Chinese medicines at the National College of Natural Medicine. She holds a particular interest in naturopathic oncology and integrative medicine, supporting patients as they navigate through cancer treatment. Malea has distinguished herself as a founding board member of the Naturopathic Advisory Board of the American Medical Student Association and as the president of the Naturopathic Medical Student Association. Malea has also worked as a research assistant at the Helfgott Research Institute, examining novel natural approaches to treating osteoporosis.
Malea MacOdrum is a 6th year student in naturopathic and Chinese medicines at the National College of Natural Medicine. She holds a particular interest in naturopathic oncology and integrative medicine, supporting patients as they navigate through cancer treatment. Malea has distinguished herself as a founding board member of the Naturopathic Advisory Board of the American Medical Student Association and as the president of the Naturopathic Medical Student Association. Malea has also worked as a research assistant at the Helfgott Research Institute, examining novel natural approaches to treating osteoporosis.
***
 Loch Chandler, ND, MSOM, LAc, is a naturopathic physician and licensed acupuncturist who has been with Providence Health & Services’ Cancer Center in their Integrative Medicine Clinic for 10 years. Dr Chandler is a 2001 graduate of the National College of Natural Medicine (NCNM) located in Portland, OR, and he is currently an adjunct faculty member at NCNM. After he graduated from NCNM, Dr Chandler was a Family Medicine resident with Dr Dickson Thom in Beaverton, OR. Dr Chandler’s specialty is helping patients diagnosed with cancer minimize side effects of treatment and maximize quality of life as they go through treatment and then afterwards with issues around survivorship. Dr Chandler has lectured around the United States on the topics of Integrative Medicine and Cancer, and Chinese Medicine and Cancer.
Loch Chandler, ND, MSOM, LAc, is a naturopathic physician and licensed acupuncturist who has been with Providence Health & Services’ Cancer Center in their Integrative Medicine Clinic for 10 years. Dr Chandler is a 2001 graduate of the National College of Natural Medicine (NCNM) located in Portland, OR, and he is currently an adjunct faculty member at NCNM. After he graduated from NCNM, Dr Chandler was a Family Medicine resident with Dr Dickson Thom in Beaverton, OR. Dr Chandler’s specialty is helping patients diagnosed with cancer minimize side effects of treatment and maximize quality of life as they go through treatment and then afterwards with issues around survivorship. Dr Chandler has lectured around the United States on the topics of Integrative Medicine and Cancer, and Chinese Medicine and Cancer.
References
- Saif MW, Reardon J. Management of oxaliplatin-induced peripheral neuropathy. Ther Clin Risk Manag. 2005;1(4):249-258.
- Cavaletti G, Tredici G, Petruccioli MG, et al. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur J Cancer. 2001;37:2457-2463.
- Boyle FM, Wheeler HR, Gillian SM. Amelioration of experimental cisplatin and paclitaxel neuropathy with glutamate. Journal of Neuro-Oncology. 1999;41(2):107-116.
- Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J Neurochem. 2008;105(2):287-295.
- Vahdat L, Papadopoulos K, Lange D, et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Cancer Res. 2001;7(5):1192-1197.
- Stubblefield MD, Vahdat LT, Balmaceda CM, et al. Glutamine as a neuroprotective agent in high-dose paclitaxel-induced peripheral neuropathy: a clinical and electrophysiologic study. Clin Oncol (R Coll Radiol). 2005;17(4):271-276.
- Loven D, Levavi H, Sabach G, et al. Long-term glutamate supplementation failed to protect against peripheral neurotoxicity of paclitaxel. Eur J Cancer Care (Engl). 2009;18(1):78-83.
- Wang WS, Lin JK, Lin TC, et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007;12(3):312-319.
- Medina MA. Glutamine and cancer. J Nutr. 2001;131(9):2539S-2542S.
- Souba WW. Glutamine and cancer. Ann Surg. 1993;218(6): 715-728.
- Eagle H. Nutritional needs of mammalian cells in tissue culture. Science. 1955;122(3168):501-514.
- Gauray K, Goel RK, Shukla M, Pandey M. Glutamine: a novel approach to chemotherapy-induced toxicity. Indian J Med Paediatr Oncol. 2012;33(1):13-20.
- Fahr MJ, Kornbluth J, Blossom S, et al. Harry M. Vars Research Award. Glutamine enhances immunoregulation of tumor growth. JPEN J Parenter Enteral Nutr. 1994;18(6):471-476.
Copyright: <a href=’https://www.123rf.com/profile_okkijan’>okkijan / 123RF Stock Photo</a>