Bradley Bush, ND
As students of the nervous system, neurologists are master conventional diagnosticians. Some continue training and become neurosurgeons. Major neurological conditions and diseases seen by neurologists include multiple sclerosis, cerebral palsy, seizures, headaches, Alzheimer’s disease, chronic fatigue syndrome, Parkinson’s disease, carpal tunnel syndrome, and neuropathy.
NDs frequently find themselves in the role of alternative health care provider for patients under a neurologist’s care. In addition to addressing the patient’s primary concerns, NDs are also asked to attend to the common comorbidities seen in chronic diseases, such as depression, insomnia, anxiety, and fatigue. In such cases, an ND’s holistic approach is not only different from that of conventional medicine, but is also sometimes even at odds with it.
The ND’s approach starts with highly detailed physical exams and laboratory workups. Although still considered novel and controversial by neurologists, neurotransmitter testing has become part of many NDs’ primary approach to neurological conditions. The premise of nervous system balancing is not new to NDs, but is actually steeped in our Nature Cure philosophy, which states that the human body is a complex and highly self-regulating life system. Disturbances that affect one body system will have an effect on other body systems.
From the time of their discovery, neurotransmitters have been associated almost exclusively with the nervous system. However, clinical and scientific evidence suggests a broader presence. The body’s immune system is in constant communication with the nervous system, with neurotransmitters playing a key role in this cross-talk. Neurotransmitters bind to immune cells and alter their activity, while immune cells release neurotransmitters once they are activated. No longer are neurotransmitters understood only as chemicals in the nervous system – they are now being redefined as neuro-immuno-transmitters that affect the body as a whole.
For the average ND, it will not come as a surprise that patients who are sick also tend to feel lethargic and find it difficult to focus or even care about most things. Our chronically ill patients often develop more severe forms of these manifestations, such as depression, anxiety, and chronic fatigue. The commonality in these circumstances resides in the increased level of circulating cytokines as a result of an activated immune system. Cytokines are to the nervous system what neurotransmitters are to the nervous system; that is, they are key communication molecules. Cytokine-induced “sickness behaviors” can be overcome once the core illness is resolved. How are cytokine-induced sickness behaviors related to neurotransmitters?
Neurotransmitter Actions in the Body
Some of these neurotransmitter-immune cell relationships are now understood:
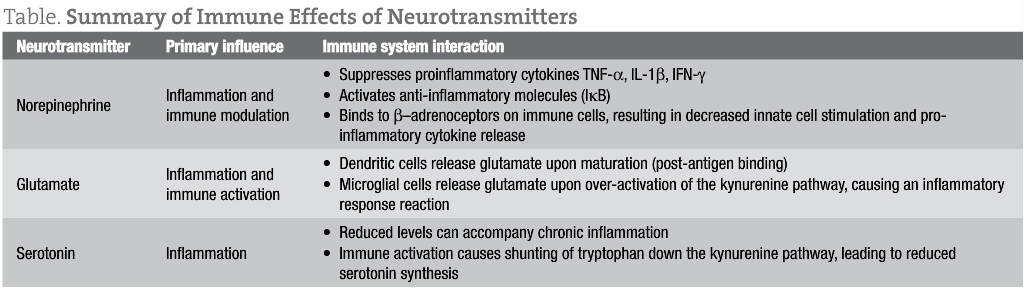
Norepinephrine (NE), an important neurotransmitter for energy and mood, also has a role in the immune system as an anti-inflammatory. NE has been shown to suppress the production of tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interferon-gamma (IFN-γ) through stimulation of receptors (β-adrenoceptors) on intestinal lymphocytes.1 NE also works in conjunction with acetylcholine to reduce inflammation via the “cholinergic anti-inflammatory pathway.”2 This pathway modulates innate immune activity through a neuronal circuit centered on cranial nerve Χ, the vagus nerve, resulting in fewer circulating pro-inflammatory cytokines and reduced inflammation.
Vagus nerve stimulation is a procedure that stimulates the vagus nerve with electrical impulses. Vagus nerve stimulation can be used to treat epilepsy.3 It has also been used to treat depression and is being studied for use in multiple sclerosis and Alzheimer’s disease.4, 5 Natural alternatives for vagus nerve stimulation include natural spinal manipulation, acupuncture, and Huperzia serrata (standardized to Huperzine-A). This extract of the Chinese herb Huperzia serrata, is a potent, highly specific, and reversible inhibitor of acetylcholinesterase (AChE),6 the enzyme that breaks down acetylcholine. This extract has been shown to modulate immune activation by directly enhancing vagal nerve activity descending from the CNS,7 and indirectly by stimulating the splenic nerve, which in turn innervates the spleen.8 In addition, it has been proposed that catecholamine release from the splenic nerve can enhance acetylcholine levels in the spleen.8,9 Personally, I have found that rheumatoid arthritis responds well to a daily combination of tyrosine (200 mg BID), for general catecholamine support, and Huperzia serrata (10-15 mg BID), probably due to a reduction in TNF-α levels.
Serotonin, known for its role in depression, has also long been suspected to play a role in the immune system. Patients with irritable bowel syndrome experience high rates of depression, which is thought to result from alterations in serotonin metabolism.10 In patients who have inflammatory diseases, depression is often a compounding factor. This has been attributed to high levels of proinflammatory cytokines, such as IL-6, IFN-γ, and TNF-α, which can lead to tryptophan depletion by activating the kynurenine and quinolinic acid pathways.11 Less tryptophan availability results in less serotonin production, and can present symptomatically in patients as irritability, anxiousness, and difficulty sleeping. I use L-tryptophan (500-2000 mg daily) and/or 5-hydroxytryptophan (5-HTP) (50-300 mg daily) with most patients who exhibit chronic inflammation.
Glutamate is a highly excitatory neurotransmitter that is neurotoxic in high concentrations. Glutamate excitotoxicity has also been linked to chronic neurodegenerative disorders such as amyotrophic lateral sclerosis, multiple sclerosis, Parkinson’s disease, and autism.12, 13
Glutamate is released from microglial cells in the nervous system and binds directly to receptors on T cells. This potentially influences cytokine expression in a concentration-dependent manner, enhancing production at lower concentrations, and decreasing production at very high concentrations.14
Glutamate is also released by dendritic cells, which are the most abundant antigen-presenting cells in the body. When dendritic cells mature and come into contact with T cells, glutamate is released.15 Clinically, this is important, since 90% of all dendritic cells are located in the intestines. My patients with “leaky gut” are often challenged by the higher concentrations of glutamate and tend to present with sleep difficulties, anxiousness (especially insidious or low-level anxiety that lasts all day), hyperactivity, trouble concentrating, and, in general, feeling stressed. Elimination diets and nutritional support appear to reduce glutamate, often leading to symptom improvements within a few weeks.
Reducing excess glutamate can be accomplished by either decreasing its production and cellular concentration or by antagonizing its receptors. The first step is to strengthen the GI tract integrity and rule out other causes of excessive dendritic cell activity, such as allergies or infections.16,17,18 Intracellular levels can be reduced with N-acetylcysteine (800-1000 mg TID). This has been shown to be effective in conditions such as autism, obsessive-compulsive disorder, addictions, and schizophrenia.19,20,21,22 Found in green tea, L-theanine is a calming amino acid by acting as a glutamate receptor antagonist, thereby reducing glutamate’s neural excitability and providing much-needed neuroprotection.23
A Whole-Body Approach
It has been shown that the body’s immune cells, including B cells, T cells, and natural killer cells, have receptors on their membranes that bind to neurotransmitters.24 Activation of these neurotransmitter receptors on immune cells will result in a number of different effects. Some neurotransmitters bind to immune cells to reduce proinflammatory cytokines; some increase production of proinflammatory cytokines; others serve roles in cytokine transport and targeting.
The interconnectedness of the human body mandates that we address the entire body rather than just one system. This is the Nature Cure approach to health. The nervous and immune systems are so intertwined that it is sometimes difficult to know where one stops and the other begins. Science has cast light on many of the biochemical nuances, but at the end of the day, even the most simplistic changes can benefit a patient.
Many traditional naturopathic approaches are effective because they impact multiple body systems at one time. Meditation and yoga have been shown to increase neurotransmitter levels and improve brain functioning.25,26 Likewise, healthy diets, clean environments, and stress reduction can help balance the nervous system.
Unfortunately, many of our patients are not in the proper place or time to make necessary lifestyle changes. They may have been exposed to toxins or infections, or may have experienced emotional trauma that keeps them from achieving their goals. When working with patients with neurological imbalances, assessing and addressing the nervous system while incorporating Nature Cure principles can be a powerful combination.
Behind the Breach in Balance
Often caused by increased production of proinflammatory cytokines, neurotransmitter imbalances can be due to many conditions, such as:
- Irritable bowel disease27,28
- Rheumatoid arthritis29
- Pathogenic infection30
- Tissue injury30
- Psychosocial stress30
- Aging30
- Oxidative stress30
Child Seizure
A 9-year old boy presents with a 2-year history of absent and (more recent) tonic-clonic seizures (3 episodes).
Meds: Phenytoin x 2 yrs, topiramate added after first tonic-clonic episode 1 month ago
Physical exam: Allergic shiners, bilateral opacified tympanic membranes
Labs ordered: IgG serum food antibody test and urinary neurotransmitter test
Treatment: 5-HTP (15 mg) and L-theanine (100 mg TID), multi-B vitamin with 5-MTHF daily, and suggested abstention from gluten and dairy
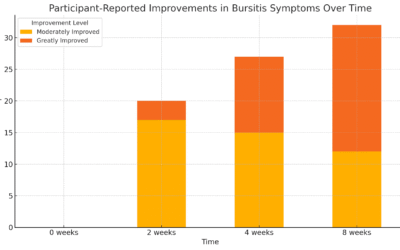
Follow up visit x 2 weeks: Patient has not experienced a tonic-colonic episode since first visit. Absent (petit mal) seizures 50% better. Mostly avoided gluten/dairy, but not fully compliant
Labs: IgG food antibody test ◊ high scores for dairy, gluten, and bananas
Urinary neurotransmitter test ◊ serotonin WNL, norepinephrine elevated, dopamine elevated, GABA elevated, glutamine elevated
Treatment: Continue with nutritional supplements and strictly avoid all highly-reactive IgG foods (recommended the entire family to follow diet)
Follow up visit x 2 months: Patient reports being free of seizures; parents are requesting to discontinue prescription medications
Note: In my experience, children with seizures tend to have normal-to-elevated urinary serotonin levels. I will still recommend 5-HTP supplementation in these cases, as long as excitatory neurotransmitters (epinephrine, norepinephrine, phenylethylamine, and glutamate) are also elevated. Increasing serotonin levels often brings dopamine levels back into balance, especially when sources of inflammation are being addressed (in this case food sensitivities).
 Bradley Bush, ND received his ND degree from National College of Naturopathic Medicine in 2000 and is currently the Director of Clinician Affairs for NeuroScience, Inc. Dr Bush specializes in neuro-endo-immune health, nutrition and infusion therapies. His focus is on addressing gastrointestinal and HPA axis disturbances in addition to nutritional deficiencies as a cornerstone of patient care. He is a co-author of the ND: Notes Science Board Review and founder and past-organizer of the annual Pharmaceutical Perspectives conference, and currently sits on the board of the Naturopathic Education and Research Consortium (NERC).
Bradley Bush, ND received his ND degree from National College of Naturopathic Medicine in 2000 and is currently the Director of Clinician Affairs for NeuroScience, Inc. Dr Bush specializes in neuro-endo-immune health, nutrition and infusion therapies. His focus is on addressing gastrointestinal and HPA axis disturbances in addition to nutritional deficiencies as a cornerstone of patient care. He is a co-author of the ND: Notes Science Board Review and founder and past-organizer of the annual Pharmaceutical Perspectives conference, and currently sits on the board of the Naturopathic Education and Research Consortium (NERC).
References
- Takayanagi Y, Osawa S, Ikuma M, et al. Norepinephrine suppresses IFN-γ and TNF-α production by murine intestinal intraepithelial lymphocytes via the β1-adrenoceptor.
- J Neuroimmonol. 2012;245(1-2):66-74.
- Pavlov VA, Wang H, Czura CJ, et al. The Cholinergic Anti-inflammatory Pathway: A Missing Link in Neuroimmunomodulation. Mol Med. 2003;9(5-8):125-134.
- Ogbonnaya S, Kaliaperumal C. Vagal nerve stimulator: Evolving trends. J Nat Sci Biol Med. 2013 Jan;4(1):8-13.
- Rado J, Janicak PG. Vagus nerve stimulation for severe depression. J Psychosoc Nurs Ment Health Serv. 2007;45(7):43-51.
- Marrosu F, Maleci A, Cocco E, et al. Vagal nerve stimulation improves cerebellar tremor and dysphagia in multiple sclerosis. Mult Scler. 2007;13(9):1200-1202.
- Tang XC, De Sarno P, Sugaya K, Giacobini E. Effect of huperzine A, a new cholinesterase inhibitor, on the central cholinergic system of the rat. J Neurosci Res. 1989;24:276-285.
- Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153-169.
- Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic anti-inflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA. 2008;105:11008-11013.
- Huston JM, Ochani M, Rosas-Ballina M., et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623-1628.
- Garvin B, Wiley JW. The role of serotonin in irritable bowel syndrome: implications for management. Curr Gastroenterol Rep. 2008;10(4):363-368.
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36(3):426-436.
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460(2):525-542.
- Purcell AE, Jeon OH, Zimmerman AW, et al. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57(9):1618-1628.
- Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988-1000.
- Pacheco R, Oliva H, Martinez-Navio JM, et al. Glutamate released by dendritic cells as a novel modulator of T cell activation. J Immunol. 2006;177(10):6695-6704.
- Berin MC,Sampson HA. Mucosal immunology of food allergy. Curr Biol. 2013 May 6;23(9):R389-400.
- Ng D, Gommerman JL. The Regulation of Immune Responses by DC Derived Type I IFN. Front Immunol. 2013;4:94.
- Gabriele L, Schiavoni G, Mattei F, et al. Novel allergic asthma model demonstrates ST2-dependent dendritic cell targeting by cypress pollen. J Allergy Clin Immunol. 2013 Apr 19. [Epub ahead of print]
- Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci. 2011;36(2):78-86.
- Hardan AY, Fung LK, Libove RA, et al. A randomized controlled pilot trial of oral N-acetylcysteine in children with autism. Biol Psychiatry. 2012;71(11):956-961.
- Afshar H, Roohafza H, Mohammad-Beigi H, et al. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2012;32(6):797-803.
- Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657.
- Oberbeck R, Schmitz D, Wilsenack K, et al. Adrenergic modulation of survival and cellular immune functions during polymicrobial sepsis. Neuroimmunomodulation. 2004;11(4):214-223.
- Kakuda T. Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction. Pharmacol Res. 2011;64(2):162-168.
- Yu X, Fumoto M, Nakatani Y, et al. Activation of the anterior prefrontal cortex and serotonergic system is associated with improvements in mood and EEG changes induced by Zen meditation practice in novices. Int J Psychophysiol. 2011;80(2):103-111.
- Streeter CC, Jensen JE, Perlmutter RM, et al. Yoga Asana sessions increase brain GABA levels: a pilot study. J Altern Complement Med. 2007;13(4):419-426.
- Chen M, Liu SZ, Zhang L. Immunoinflammation and functional gastrointestinal disorders. Saudi journal of gastroenterology. 2012;18(4):225-229.
- Brynskov J, Foegh P, Pedersen G, et al. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut. 2002;51(1):37-43.
- Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol. 2006;18(3):277-281.
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226-238