Arlene Donar, ND
Two female patients, 21 and 24 years old, recently presented to my office with idiopathic long-term cessation of menses. Secondary amenorrhea ranged from 15 months in the 21-year-old to 3+ years in the 24-year-old. Menopausal symptoms such as vaginal dryness and weight gain were evident.
Treatments that included herbal tinctures, vitamin and mineral supplementation, detoxification, dietary and lifestyle modification and bio-identical hormone replacement in conjunction with simultaneous biweekly acupuncture sessions did not result in the resumption of menstruation.
Had I considered an autoimmune etiology earlier on in their treatment with an emphasis on immune modulating therapies, I might have spared these young women the prolonged investment of time, expense and emotional distress. The involvement of autoimmune mechanisms resulting in ovarian insufficiency and premature ovarian failure (POF) is not often considered in a working differential diagnosis. It is a relatively uncommon condition, and there is no typical menstrual history for women with POF. Cessation of menses is frequently attributed to other conditions without recommending in-depth diagnostic testing. Studies have indicated that a majority of women with complaints of idiopathic amenorrhea or other disturbances in menstrual patterns reported visiting a clinician’s office three or more times before laboratory testing was undertaken to determine a diagnosis. More than 50% stated that they had seen three or more clinicians before diagnosis, and 25% sought care for longer than five years before the diagnosis of POF was established. A delay in the evaluation of POF and misdirected treatments may have significant consequences for these young women, such as early onset of osteopenia (Alzubaidi et al., 2002). More importantly, a refocus of the direction of treatment with a transition to immune-modulating therapies may maintain and/or restore ovarian function and fertility.
Determining a clear-cut diagnosis of POF can be a daunting task. At this time, there is no proven test to confirm that a woman has POF on an autoimmune basis. In a study by Novosad et al. (2003), one-half of young women with POF were found to have ovarian antibodies; however, 31% of the normal control groups also had ovarian antibodies using current commercial laboratory testing. Since the target of the autoimmune response is so varied, the specificity of ovarian antibodies has come into question. Targets include the ovarian follicle (both the granulosa and thecal layers), the zona pellucida, corpus luteum, the oocyte in general as well as gonadotropin receptors. Often, therapeutic strategies must be determined by clinical observation, detailed patient/familial history, associated autoimmune biological markers and sex steroid deficiencies, particularly when other therapies have proven uneventful.
What is Autoimmune POF?
Autoimmunity is an established mechanism of ovarian failure. One percent of women by age 40 spontaneously develop POF. It is characterized by amenorrhea, infertility, sex steroid deficiency and elevated serum FSH levels (>10 IU/I) (Kalantaridou et al., 1999). Onset of autoimmune POF usually occurs in childhood, adolescence or early adulthood (Kauffman et al., 2003). Puberty and the presence of increased serum gonadotropins appear to enhance the autoimmune response in experimental models of POF (Melner and Feltus, 1999).
Association with Autoimmune Diseases
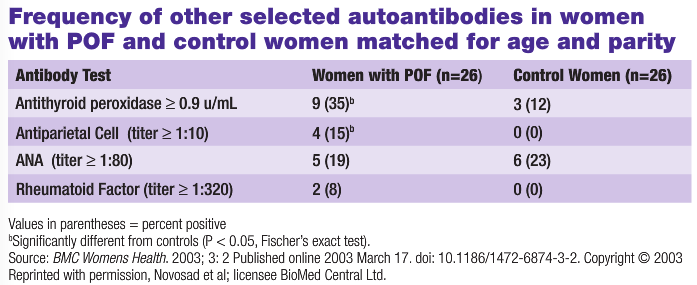
It has long been recognized that POF could be associated with nearly all organ-specific autoimmune diseases. An early association with adrenal autoimmunity and atrophic ovaries was suggested in a study by Duff and Bernstein in 1933. Since then, various studies further linked POF with Hashimoto’s thyroiditis, Grave’s disease, multiple sclerosis, myasthenia, rheumatoid arthritis, pernicious anemia, vitiligo, Crohn’s disease and celiac disease; as well as non-organ specific disorders, such as systemic lupus erythematosus and idiopathic thrombocytopenia. Among all autoimmune diseases associated with POF, thyroid disorders have the closest association, and have been detected in 12%-33% of patients. In 18% of these cases, there is a family history of autoimmune thyroid disease (Forges, 2004). The estimated incidence of the association of autoimmune disease with POF is 17%, and a significant number of patients carry at least one class of autoantibody (Ishizuka et al., 1999). In the 2003 study by Novosad et al., women with POF were significantly more likely to have positive thyroid peroxidase and parietal cell autoantibodies than women with normal ovarian function (see Table 1).
Ishizuka et al. (1999) detected anti-nuclear antibodies (ANA) more frequently in patients with POF with normal karyotypes than in control amenorrheic patients. ANA was found in 77% (10/13) of POF patients with normal karyotypes who developed amenorrhea at or younger than the age of 30 years, but not in patients who developed amenorrhea later in life (Ishizuka et al., 1999).
More recently, studies have focused on the cellular component of the autoimmune response. As in other autoimmune diseases, the absolute count of peripheral blood lymphocytes, particularly CD4+ T cells, was increased in patients with POF (Mignot et al., 1989). Low levels of CD8+/CD57+ T cells and overall increase in the CD4+/CD8+ ratio has also been noted. Higher levels of activated T cells were shown in 35%-50% of patients with a higher expression of the MHC class II molecules, especially HLA-DR on their T lymphocytes than in healthy control women. Also, the activity and number of natural killer cells was reduced (Forges, 2004).
 Subclinical Autoimmune POF?
Subclinical Autoimmune POF?
Wheatcroft et al. (1997) studied a group of young women (<40 years old) with regular menses, unexplained infertility and elevated basal serum FSH to establish the frequency of autoimmune markers. No circulating T cell abnormalities were found; however, they did discover significantly elevated levels of terminal complement complexes (TCC) and C3a. TCC are the products of complement activation released following membrane attack, and serum concentrations are elevated in numerous autoimmune and inflammatory conditions. C3a is a breakdown product of the complement cascade, and is also evident during acute inflammatory reactions (Wheatcroft et al., 1997). This study suggests that elevated concentrations of these markers indicate the presence of an inflammatory process, which is significantly higher in the above-mentioned group. This may be indicative of a group of women who present with regular menstrual cycles and unexplained infertility without clinical evidence of autoimmune diseases that have a subclinical form or early onset of autoimmune POF. Autoimmune POF is a relatively uncommon condition. Due to the varying menstrual histories presented by women with POF, this often is not included in our working differential diagnoses. This, in conjunction with poor specificity of laboratory tests currently available, often delays diagnosis and appropriate immune-modulating treatment. Often, therapeutic strategies must be determined by clinical observation, detailed patient/familial history, associated autoimmune biological markers and sex steroid deficiencies, particularly when other therapies have not produced the target goal of resumption of menses. Earlier identification of women with autoimmune POF presents the opportunity to possibly maintain and/or restore ovarian function, also sparing these patients years of office visits, misdiagnosis, expense and emotional distress.

Arlene B. Donar, ND earned her naturopathic degree from the University of Bridgeport College of Naturopathic Medicine. She is board certified and licensed as an ND in Connecticut. Dr. Donar also holds a Master of arts degree in speech and language pathology, and has worked as both a private consultant and adjunct professor in speech communications. Dr. Donar is the medical director of the supplement manufacturer Heartguardian. She maintains a private practice in Manhattan, working primarily with clients with chronic health conditions, and lectures locally on prevention and treatment of disease using botanical medicine and clinical nutrition.
References
Alzubaidi MD et al: Meeting the needs of young women with spontaneous premature ovarian failure, Obs & Gy.,99:720-725, 2002.
Kalantaridou SN et al: Treatment of autoimmune premature ovarian failure: case report, Hum Rep 14:1777-1782, 1999.
Kauffman RP, Castracane V: Premature ovarian failure associated with autoimmune polyglandular syndrome: pathophysiological mechanisms and future fertility, Journal of Women’s Health 12:513-520, 2003.
Melner MH, Feltus FA: Editorial: autoimmune premature ovarian failure – endocrine aspects of a T cell disease, Endocr 140:3401-3403, 1999.
ForgesT et al: Autoimmunity and antigenic targets in ovarian pathology, Hum Rep Update 10:163-175, 2004.
Ishizuka B et al: Anti-nuclear antibodies in patients with premature ovarian failure, Hum Rep 14:70-75, 1999.
Novosad JA et al: Ovarian antibodies as detected by indirect immunofluorescence are unreliable in the diagnosis of autoimmune premature ovarian failure: a controlled evaluation, BMC Wom Health 3:online, 2003.
Wheatcroft NJ et al: Is subclinical ovarian failure an autoimmune disease?, Hum Rep 12:244-249, 1997.


