Naturopathic Perspective
Andrea Gruszecki, ND
Many aspects of human physiology are influenced by levels of steroid sex hormones, and the immune system is no exception. A thorough evaluation and understanding of an autoimmune patient’s native hormone metabolism may provide insight and therapeutic options. Hormone effects are dimorphic; that is, the effect of a hormone varies based on the gender of the individual.1 This dimorphic nature of human immune responses predispose women toward autoimmune disorders and men toward cancer.2 Women, for example, tend to have enhanced antibody production, better resistance to infection, and are less susceptible to viral infections compared to men.2 The reason for these effects may be evolutionary: the female may require a stronger immune system to protect and bear offspring and to “program” the immunity of the next generation.
Men, on the other hand, may be partially protected from autoimmune conditions due to their higher levels of androgens. Dehydroepiandrosterone (DHEA), testosterone, and dihydrotestosterone (DHT) tend to confer immunosuppressive qualities and have been shown to downregulate immune responses in both autoimmune patients and controls.3 Progesterone is usually considered immunosuppressive; however, several studies indicate that the effects of progesterone on some immune cells, such as dendritic cells, may be complicated by prior hormone exposures.4 It is the estrogens, due to their bimodal effects, that may have the most varied impact on autoimmune disorders. In fact, an estrogen metabolite has recently been found to be associated with some autoimmune conditions.5
Hormonal Effects on Immune Activity
The effects of the estrogen, 17β-estradiol (E2), on the immune system are bimodal; cellular response is different when E2 levels are too low (proinflammatory) vs when they are too high.6 Estradiol downregulates the genetic expression of the autoimmune regulator protein, which likely contributes to the higher predominance of autoimmune conditions in women, and E2 also upregulates DNA methyltransferase-3b to further alter epigenetic programming.3 Estrogen regulation of the immune system determines the number, differentiation, and function of peripheral neutrophils, T-cells, B-cells, macrophages, and nervous system microglia. Within the immune cells, estrogens (primarily E2) regulate the genes that control the immune responses, including the expression of tumor necrosis factor-alpha (TNFα), interleukins, interferon-gamma (IFNγ) B-cell immunoglobulins, and other cytokines.7
Estrogen effects may be a direct response to the binding of estrogen (or another ligand) to one of the 2 estrogen receptors, ERα or ERβ. Post-translational modifications (phosphorylation, glycosylation, acetylation, nitrosylation, etc) of estrogen receptors results in ligand-independent, or indirect, signaling. A variety of local factors, including differential distribution of the 2 ER receptors in cells and tissues, inherited variations in the receptors, and the local tissue profile (inflammatory vs tolerant) may all influence estrogen receptor signaling. ERα is expressed by most of the cells in the immune system, whereas ERβ is variably expressed by most immune cells. B-cells express more ERβ, while T-lymphocytes express more ERα.7
The balance of signals received by developing immune cells through their androgen, progesterone, and estrogen receptors determines, in part, the populations of various T-cell subtypes, including T-regulatory cells (T-regs), Th1 lymphocytes, Th2 lymphocytes, and Th17 lymphocytes.1 Th1 cells produce IFNγ, interleukin (IL)-2 and TNFβ, and promote cell-mediated immunity and phagocyte-dependent inflammation. Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13; in so doing, they evoke strong antibody responses (including IgE) and eosinophil accumulation.8 Different autoimmune conditions have comparatively increased populations of dysregulated Th1 or Th2 cells, with or without elevated Th17 cells. In some autoimmune conditions, such as rheumatoid arthritis (RA), the immune cells in synovial tissues may change over the course of 2 years, evolving from a Th2-dominant profile to a Th1-dominant profile.9
While knowing the status of a patient’s immune profile is essential for the provision of good patient care, it may be just as necessary to evaluate the patient’s native hormone metabolism and to monitor the patient during any hormone therapy intervention. Recent evidence of metabolic disruptions in steroid hormone metabolism, due to inflammation and disease processes, can often be inferred from a urine hormone analysis.10
Figure 1. Low Androgens, Higher Estrogens
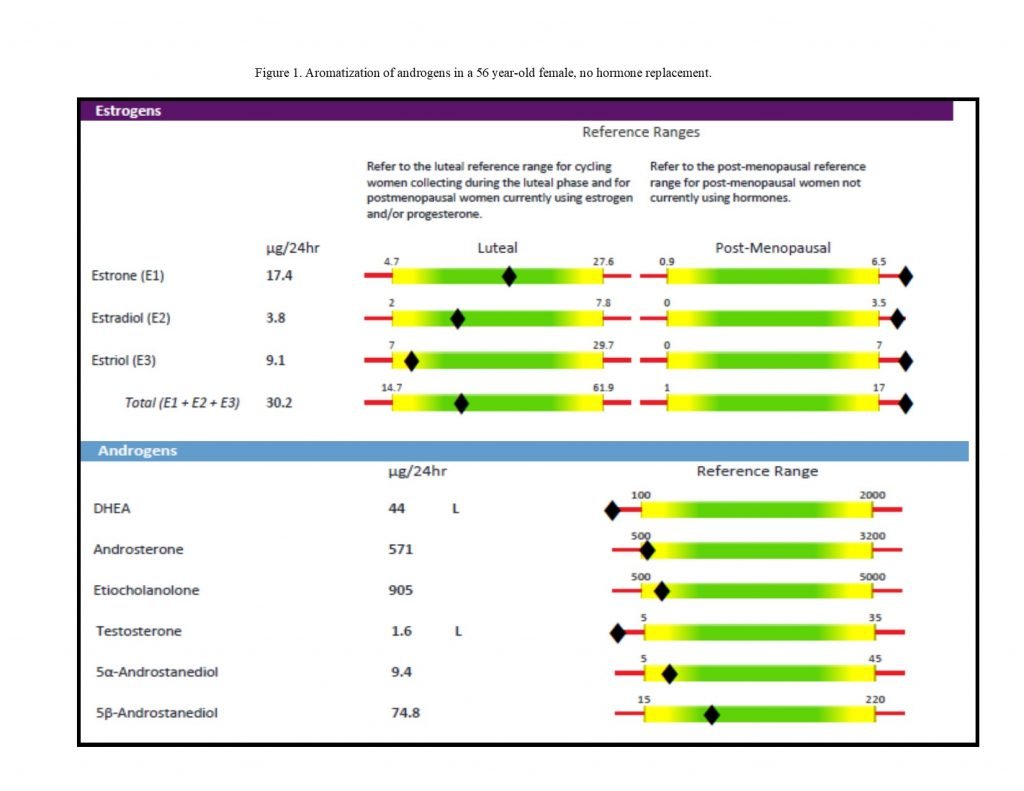
Th2-Dominant Autoimmunity
A common presenting pattern in autoimmune individuals can be seen in Figure 1.5 The androgen levels, particularly that of DHEA, are lower than expected, and the estrogens are higher by comparison. Although the effects of estrogen and other hormones on immune activity are complex and variable, much of the available evidence suggests that estrogens mostly stimulate Th2 and inhibit Th1.11,12 Androgens are converted into estrogens by the aromatase enzyme (see Figure 2). Current evidence indicates that peripheral aromatase activity may be increased by inflammatory cytokines, such as TNFα and IL-6. Excess estrogen from aromatization may exacerbate symptoms and inflammation in patients with Th2-predominant autoimmune conditions.12 Estrogen-progesterone replacement therapies may be used in patients with RA and systemic lupus erythematosus (SLE) patients, but should be low-dose and carefully balanced in SLE patients to prevent flare-ups.1
Figure 2. Hormone Metabolism Pathways
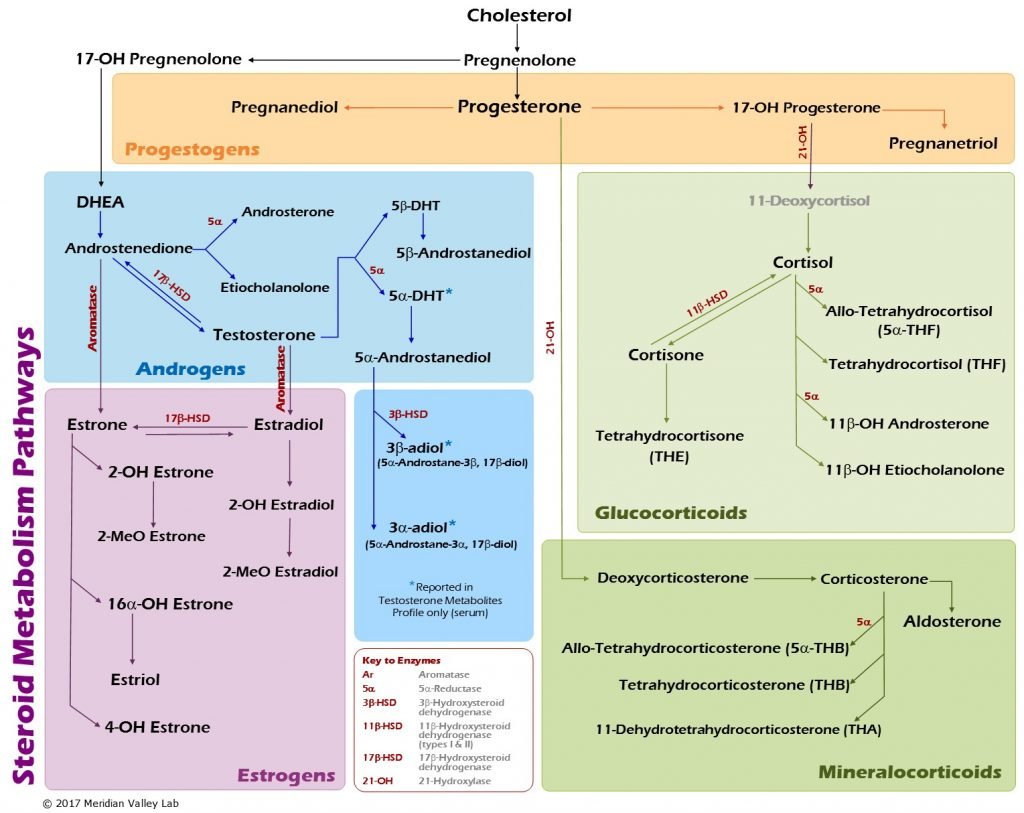
Estrogen Metabolism: Modulation
The evaluation of estrogen metabolites may be particularly important for patients with certain autoimmune conditions, such as RA and SLE.5 The 16α-hydroxyestrogens bind estrogen receptors tightly and the binding does not permit downregulation of the receptor; this gives the 16α-metabolites estradiol-like effects (Figure 3).13 Both RA and SLE patients may present with low urinary 2-hydroxyestrogens and an increased 2:16α-hydroxyestrogen ratio.14 Multiple strategies may be used to positively shift this ratio, and evaluations may be done pre- and post-intervention to document progress:
- Exercise has been shown to increase the urinary levels of 2-hydroxyestrogens and decrease urinary 16α-metabolites in both pre- and postmenopausal women15,16
- Resveratrol (1 g daily with meals for 12 weeks) has been shown to increase urinary 2-hydroxyestrone by up to 73%17
- Indole-3-carbinol (200-400 mg daily) has been shown to improve urinary hydroxyestrogen ratios in a dose-dependent manner18
- 3,3′-Diindolylmethane (DIM) (108 mg daily for 1 month) has been shown to significantly increase urinary 2-hydroxyestrogen levels and improve the 2:16α-hydroxyestrogen ratio by up to 47%19
- Increased intake of lignans in fiber and flaxseed has been shown to improve urinary levels of 2-hydroxyestrogens and hydroxyestrogen ratios20
- Increased dietary intake of fruits and vegetables (particularly cruciferous) has been shown to improve urinary 2-hydroxyestrogens and hydroxyestrogen ratios20,21
Figure 3. Estrogen Metabolite Ratios
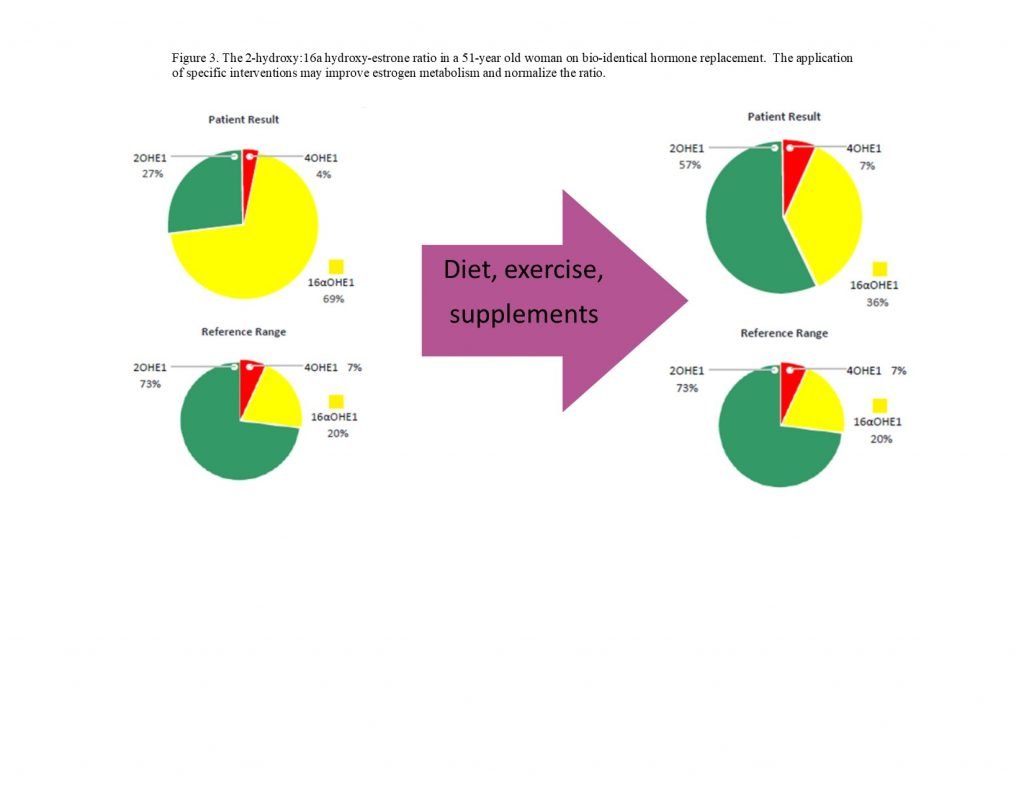
Evaluating 4-hydroxyestrogen levels can also provide valuable clinical information, as higher levels of 4-hydroxyestrogens may be converted into quinone molecules that can directly damage DNA and/or increase oxidative stress.22 Reducing the risks associated with 4-hydroxyestrogens involves supporting detoxification methyltransferase enzymes with magnesium and vitamin B6, supporting glutathione enzymes with selenium, and providing additional antioxidants, such as resveratrol and N-acetylcysteine.22,23
Th1-Dominant Autoimmunity
For patients with Th1-dominant autoimmune disorders, lower levels of estrogens and androgens (Figure 4) may exacerbate inflammation and contribute to disease progression. There is some evidence, for example, that in women with multiple sclerosis (MS), follicular estradiol is lower in cycling women,24 and that MS worsens after menopause in many women.25 Certain autoimmune conditions often improve during pregnancy when estrogen levels are higher, such as MS, thyroiditis, rheumatoid arthritis, uveitis, and psoriasis.26 Increasing the level of estrogens, particularly via estriol supplementation, may have anti-inflammatory and neuroprotective effects in Th1-dominant autoimmune conditions such as MS.26 A deficiency in iodine and/or selenium has been shown to decrease cytochrome P450 enzyme activity; iodine, in particular, is required by cytochrome P450 3A4 in the local conversion of 16a metabolites into estriol.23,27,28
Figure 4. Low Endogenous Hormone Levels
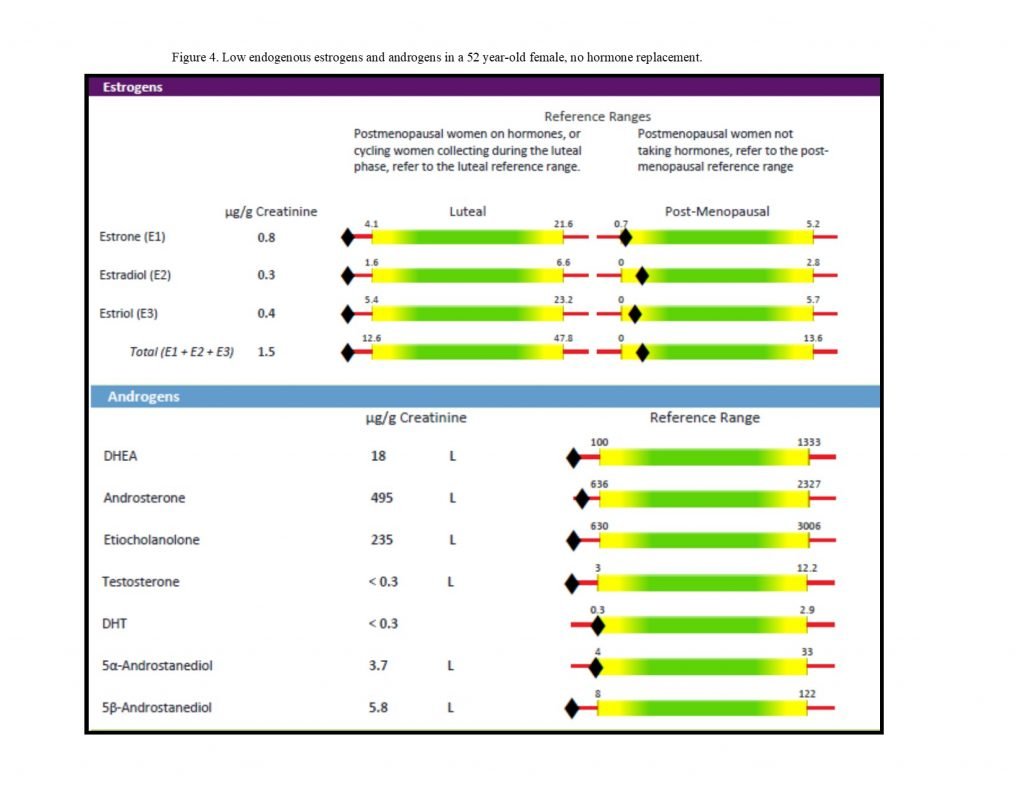
Supporting Hormone Balance
The correction of low hormone levels in mature patients, ie, post-menopause or post-andropause, is fairly straightforward. Human trials have begun in which female MS patients are administered doses of estriol (E3) and progesterone that mimic the third trimester of pregnancy, with the goal of inducing a shift from Th1- to Th2-dominant immunity.26 However, in younger patients, particularly cycling women with regular menses, supplementation with estradiol may be contraindicated due to increased cancer risks; thus, longer-term human trials are needed to fully assess the safety of high-dose estriol. Meanwhile, the daily provision of certain nutrients may improve endogenous sex and glucocorticoid hormone production in younger, cycling patients:
- Vitamin A, up to 10 000 IU for non-pregnant adults
- Retinoic acid may regulate the activity of estrogen metabolism enzymes29
- Vitamin B3, up to 16 mg30
- Precursor for NAD(P)+, a necessary cofactor for enzymes of estrogen metabolism; also supports mitochondrial hormone synthesis enzymes
- Vitamin D, 800-1000 IU (adjust dose as needed; check serum levels)31
- Regulates the expression of hundreds of genes; higher levels also associated with higher levels of testosterone and sex hormone-binding globulin in men
- Vitamin K2, 25-50 µg32,33
- Ligand for nuclear receptors that upregulate cytochrome P450 enzymes CYP1, CYP2, CYP3, and CYP4
- Boron, 3-9 mg34
- Increases synthesized levels of vitamin D, sex hormones, and thyroid in human studies
- Mineral cofactors for enzyme function35,36
- Iodine, 1 mg or per clinical indications
- Iron (if anemic)
- Magnesium, 350 mg or to bowel tolerance
- Manganese, up to 11 mg
- Selenium, 200-400 µg
- Zinc, 8-40 mg (balance with copper)
These nutritional interventions may promote not only primary, but also local (intracrine), synthesis in tissues such as the central nervous system, bones, lungs, skin, and gastrointestinal systems. Intracrine synthesis is essential after menopause, and improving estrogen metabolism may provide further benefits for any patient suffering from localized or systemic inflammation.35 Because so many aspects of human physiology, including the immune system, are influenced by levels of steroid sex hormones, a thorough evaluation and understanding of an autoimmune patient’s native hormone metabolism may provide additional insight and therapeutic opportunities to restore health.
References:
- Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol. 2018;9:2279.
- Taneja V. Sex Hormones Determine Immune Response. Front Immunol. 2018;9:1931.
- Edwards M, Dai R, Ahmed SA. Our Environment Shapes Us: The Importance of Environment and Sex Differences in Regulation of Autoantibody Production. Front Immunol. 2018;9:478.
- Larson TA. Sex Steroids, Adult Neurogenesis, and Inflammation in CNS Homeostasis, Degeneration, and Repair. Front Endocrinol (Lausanne). 2018;9:205.
- Cutolo M, Sulli A, Straub RH. Estrogen metabolism and autoimmunity. Autoimmun Rev. 2012;11(6-7):A460-A464.
- Straub RH. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res Ther. 2014;16(1):203.
- Khan D, Ansar Ahmed S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Front Immunol. 2016;6:635.
- Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol. 2000;85(1):9-18.
- Aarvak T, Chabaud M, Thoen J, et al. Changes in the Th1 or Th2 cytokine dominance in the synovium of rheumatoid arthritis (RA): a kinetic study of the Th subsets in one unusual RA patient. Rheumatology (Oxford). 2000;39(5):513-522.
- Michaud DS, Manson JE, Spiegleman D, et al. Reproducibility of plasma and urinary sex hormone levels in premenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1059-1064.
- Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3(1):97-104.
- Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173(3):600-609.
- Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Circulating 2-hydroxy and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2029-2035.
- Weidler C, Härle P, Schedel J, et al. Patients with rheumatoid arthritis and systemic lupus erythematosus have increased renal excretion of mitogenic estrogens in relation to endogenous antiestrogens. J Rheumatol. 2004;31(3):489-494.
- Matthews CE, Fortner RT, Xu X, et al. Association between physical activity and urinary estrogens and estrogen metabolites in premenopausal women. J Clin Endocrinol Metab. 2012;97(10):3724-3733.
- Dallal CM, Brinton LA, Matthews CE, et al. Association of Active and Sedentary Behaviors with Postmenopausal Estrogen Metabolism. Med Sci Sports Exerc. 2016;48(3):439-448.
- Chow HH, Garland LL, Heckman-Stoddard BM, et al. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med. 2014;12:223.
- Bell MC, Crowley-Nowick P, Bradlow HL, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78(2):123-129.
- Dalessandri KM, Firestone GL, Fitch MD, et al. Pilot study: effect of 3,3′-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50(2):161-167.
- Lord RS, Bongiovanni B, Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7(2):112-129.
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis.Pharmacol Res. 2007;55(3):224-236.
- Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention. Clin Transl Med. 2016;5(1):12.
- Baker RD, Baker SS, LaRosa K, et al. Selenium regulation of glutathione peroxidase in human hepatoma cell line Hep3B. Arch Biochem Biophys. 1993;304(1):53-57.
- Foroughipour A, Norbakhsh V, Najafabadi SH, Meamar R. Evaluating sex hormone levels in reproductive age women with multiple sclerosis and their relationship with disease severity. J Res Med Sci. 2012;17(9):882-885.
- Bove R, Healy BC, Musallam A, et al. Exploration of changes in disability after menopause in a longitudinal multiple sclerosis cohort. Mult Scler. 2016;22(7):935-943.
- Ali ES, Mangold C, Peiris AN. Estriol: emerging clinical benefits. Menopause. 2017;24(9):1081-1085.
- Erkekoglu P, Giray BK, Caglayan A, Hincal F. Selenium and/or iodine deficiency alters hepatic xenobiotic metabolizing enzyme activities in rats. J Trace Elem Med Biol. 2012;26(1):36-41.
- Grover S, Talwar P, Baghel R, et al. Genetic variability in estrogen disposition: Potential clinical implications for neuropsychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(8):1391-1410.
- Cheng YH, Yin P, Xue Q, et al. Retinoic acid (RA) regulates 17beta-hydroxysteroid dehydrogenase type 2 expression in endometrium: interaction of RA receptors with specificity protein (SP) 1/SP3 for estradiol metabolism. J Clin Endocrinol Metab. 2008;93(5):1915-1923.
- Chapman JC, Polanco JR, Min S, Michael SD. Mitochondrial 3 beta-hydroxysteroid dehydrogenase (HSD) is essential for the synthesis of progesterone by corpora lutea: an hypothesis. Reprod Biol Endocrinol. 2005;3:11.
- Anic GM, Albanes D, Rohrmann S, et al. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin Endocrinol. 2016;85(2):258-266.
- Zhou C, Verma S, Blumberg B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal. 2009;7:e001.
- Lasemi R, Kundi M, Moghadam NB, et al. Vitamin K2 in multiple sclerosis patients. Wien Klin Wochenschr. 2018;130(9-10):307-313.
- Eskin N. Boron: An Overlooked Micronutrient that Plays an Important Role in Human Physiology. Vitam Miner. 2015;4:e135.
- Linus Pauling Institute. Micronutrient Information Center. Minerals. Oregon State University Web site. https://lpi.oregonstate.edu/mic/minerals. Accessed November 19, 2018.
- Konings G, Brentjens L, Delvoux B, et al. Intracrine Regulation of Estrogen and Other Sex Steroid Levels in Endometrium and Non-gynecological Tissues; Pathology, Physiology, and Drug Discovery. Front Pharmacol. 2018;9:940.
 Andrea Gruszecki, ND, received her doctorate in Naturopathy from Southwest College of Naturopathic Medicine in 2000. Trained as a radiologic technologist and Army medic, she spent the years prior to graduation working in urgent care and hospital settings, gaining valuable clinical experience. Dr Gruszecki is a member of the Consulting Department at Meridian Valley Laboratory, where she provides interpretive assistance with laboratory results, writes interpretations, and creates conference presentations.
Andrea Gruszecki, ND, received her doctorate in Naturopathy from Southwest College of Naturopathic Medicine in 2000. Trained as a radiologic technologist and Army medic, she spent the years prior to graduation working in urgent care and hospital settings, gaining valuable clinical experience. Dr Gruszecki is a member of the Consulting Department at Meridian Valley Laboratory, where she provides interpretive assistance with laboratory results, writes interpretations, and creates conference presentations.


