A comprehensive review of how targeted oral probiotics like Streptococcus salivarius K12 support the oral microbiome, improve ENT health, and may offer broader immune benefits in children.*
Jamie Oskin, ND, DTBRm, DHANP
Abstract
Children’s immune resilience is closely linked to the health of their oral and upper respiratory microbiomes. Disruptions to this delicate ecosystem—from antibiotics, diet, or chronic infections—can increase susceptibility to inflammation, dysbiosis, and recurrent ENT (ear, nose, and throat) issues. This article explores how targeted oral probiotics, particularly Streptococcus salivarius K12, can help restore balance within the oral microbiome and provide broad-spectrum support for ENT and immune health in pediatric populations. Drawing on more than a decade of clinical experience and over 100 peer-reviewed studies, Dr. Oskin reviews the mechanisms of action, safety, and efficacy of S. salivarius K12, including its impact on recurrent infections, halitosis, dental health, and immune modulation. Additional attention is given to related strains like S. salivarius M18 and emerging research on oral microbiome restoration in autoimmune conditions. Practical dosing, product considerations, and real-world clinical outcomes are shared to help clinicians integrate oral probiotic therapy into pediatric care with confidence.
INTRODUCTION
The oral microbiome, along with connected microbiomes of the ears, nose, and throat, can be disrupted by a variety of environmental factors, which can make a child more susceptible to inflammation, dysbiosis, and recurrent infections. Some of the leading causes of dysbiosis in the oral microbiome include the overuse of antibiotics, poor oral hygiene, a diet high in refined carbohydrates, antiseptic mouthwashes, mouth breathing during sleep, as well as chronic or recurrent viral or bacterial infections. Supporting children’s (and adults’) oral microbiome helps to support their natural immune system defenses, maintain upper respiratory tract health, and support ear, nose, and throat immune health.
Lifestyle factors that may improve children’s health include limiting overuse of antibiotics, improving oral hygiene, healthy nutrition that avoids sugars and refined carbohydrates, and reducing mouth breathing. In addition, studies have now identified some key strains of beneficial bacteria that can aid in supporting healthy levels of bacteria in the ears, mouth, and throat.* Several key probiotics have been researched to improve the oral microbiome, though a few targeted strains seem to indicate the most promise in the literature.
The most prominent targeted probiotic strain that originates in the oral microbiome, which is beneficial, is called Streptococcus salivarius K12. This strain of probiotic contains Bacteriocin-Like Inhibitory Substances (BLIS). These are natural peptides that actively target bad bacteria in the ears, nose, mouth, and throat of young children and adults as well as promote and support fresh breath by helping to maintain a healthy level of beneficial bacteria in the mouth*. S. salivarius K12 seems to work via mechanisms to colonize1 the upper respiratory tract and thereby competitively exclude pathogenic strains of bacteria, provide antimicrobial inhibition, and immune modulation2-4, both locally and systemically.*
For over a decade, I have been using oral probiotic products containing S. salivarius K12 in my practice, which is predominantly pediatric-focused, in order to support children’s immune system and oral microbiome. My own clinical use is supported by over 115 research studies that have been published, with clinical trials ranging from 30 to 180-day courses with the S. salivarius K12 probiotic strain. In this article, I will give a summary review of some of the key findings in the research related to S. salivarius K12, as well as some other promising strains of beneficial probiotics.
UPPER RESPIRATORY TRACT
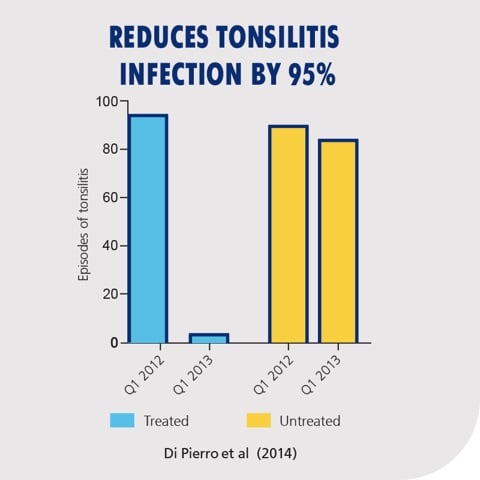
Several clinical trials have found S. salivarius K12 to be helpful for children who have a history of recurrent tonsillitis and otitis media (Figure 1). Children who completed a 90-day trial with a probiotic containing S. salivarius K12 showed a 95% reduction in tonsillitis episodes compared to the untreated control group.*5 Additionally, the subjects in the treatment arm of the study showed an 80% decrease in incidence of oral viral infections, while there was no difference in the control group*. The study also found a significant reduction in the total number of days under antibiotic (30 compared to 900) and antipyretic (16 compared to 228) treatment compared to the control group, as well as a reduction in days absent from school (and work for the parents; 16 compared to 228).* This study found, as has been repeatedly found in other studies, that the probiotic was well tolerated with no side effects.
Figure 1: Subjects treated with S. salivarius K12 showed a 95% reduction in tonsillitis episodes compared to the untreated control group. Adapted from: Di Pierro et al. (2014).
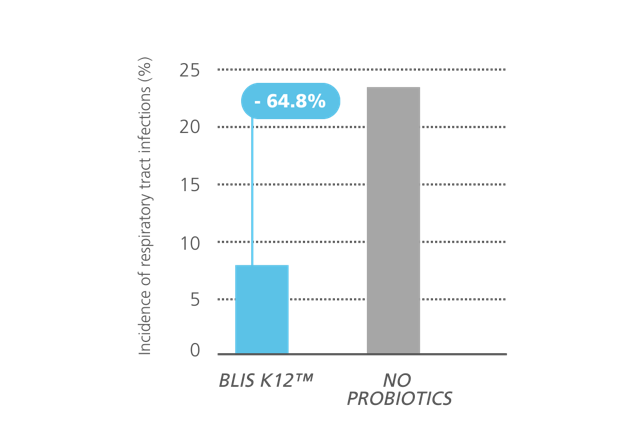
In a 30-day trial of adults, a 2021 study reduced the incidence of respiratory tract infections by 64.8% when taking S. salivarius K12.* Further, it showed that the duration of respiratory tract infection symptoms and the days absent from work were reduced by 78% and 95.5% respectively.*6
Figure 2: Subjects who received daily Streptococcus salivarius K12 supplementation experienced a 64.8% reduction in respiratory infections compared to the control group. Adapted from: Wang et al., 2021.
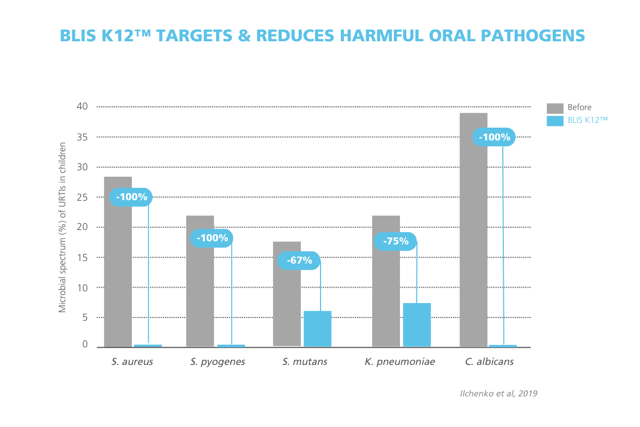
A 2019 study in children observed significant decreases in the presence of S. aureus, S. pyogenes, S. mutans, K. pneumoniae, and C. albicans, with complete elimination in several species when taking S. salivarius K12.*7
Figure 3: Reduction in key oral and upper respiratory tract pathogens in children following Streptococcus salivarius K12 supplementation. Significant decreases were observed in the presence of S. aureus, S. pyogenes, S. mutans, K. pneumoniae, and C. albicans, with complete elimination in several species. Adapted from: Ilchenko et al., 2019.
A 2016 study evaluated children aged 3 to 10 for 90 days, taking S. salivarius K12.8 In this study, the children who completed the course of S. salivarius K12 had a reduction of 90% in episodes of streptococcal pharyngeal infection compared to the previous year.* The treated children also had significantly fewer episodes of tracheitis, viral pharyngitis, rhinitis, flu, laryngitis, acute otitis media, and enteritis compared to the untreated group.* Another preliminary study from 2021 found that children taking S. salivarius K12 for 90 days had a lower rate of COVID-19 infection.*9
Figure 4: Reduction in viral pharyngitis, viral rhinitis, and flu-like symptoms following Streptococcus salivarius K12 supplementation. Compared to baseline, significant decreases were observed in the number of episodes per subject. Adapted from: Di Pierro et al., 2016.
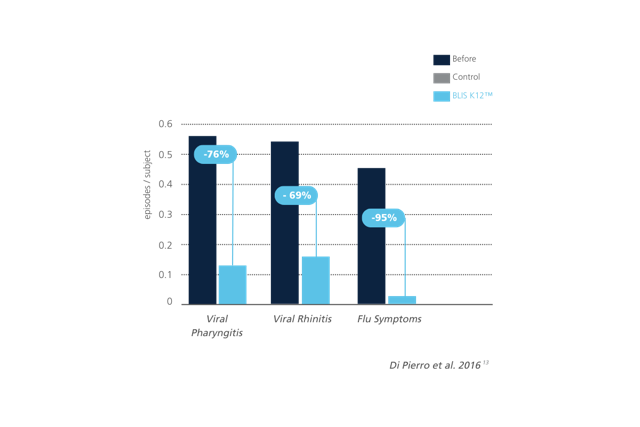
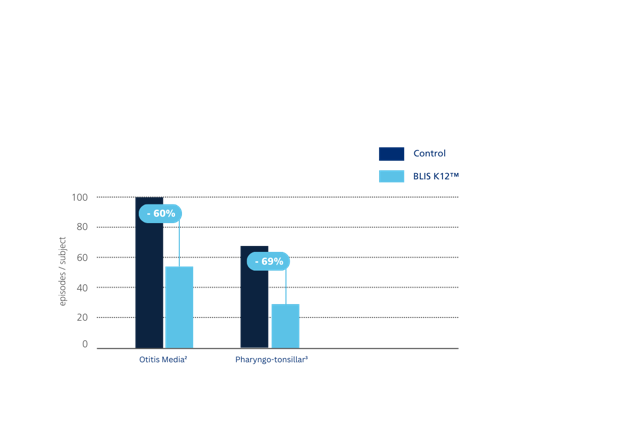
Figure 5: Streptococcus salivarius K12 significantly reduced episodes of otitis media and pharyngo-tonsillar infections in children compared to controls. Incidence of otitis media was reduced by 60% and pharyngo-tonsillar infections by 69%. Adapted from: Di Pierro et al., 2016.
A 2022 study using S. salivarius K12 in school children with recurrent respiratory tract infections found that it helped to reduce upper respiratory tract infections during peak cold season, which resulted in reduced days of using antibiotics and antiviral drugs, and reduced children’s sick days from school.* It reduced parents’ absent days from work.* They also found that those who became ill experienced a shortened course of respiratory infections.*10 In another study, when S. salivarius K12 was given for one month after antibiotics for children with a Group A beta hemolytic S. pyogenes throat infection (GAS), those children had a significant reduction in GAS prevalence, carriage, and throat infections by limiting the healthy children from acquiring it.*11
BREATH, HALITOSIS, TEETH & GUM HEALTH
Microbial dysbiosis can contribute to halitosis when oral bacteria produce malodorous compounds such as volatile sulfur compounds (VSC), valeric acid, butyric acid, and putrescine. S. salivarius K12 has been found to reduce VSC and inhibit halitosis-causing bacteria. In a 2006 study of adults who used mouthwash followed by a S. salivarius K12 lozenge, researchers found that after one week, 85% of the group taking the probiotic had greater than 100 ppb VSC reduction in the breath compared to only 30% in the placebo group.*12 Another study found improvements in bad breath in children.*13
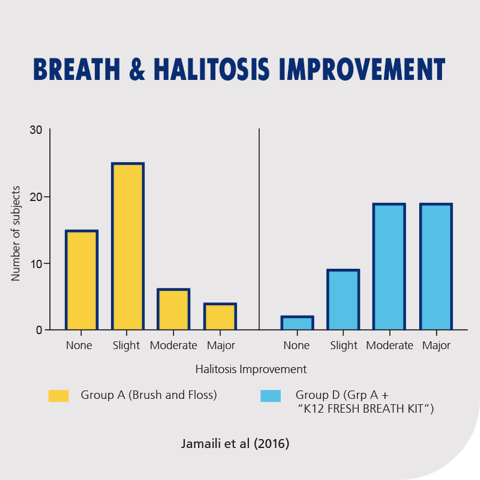
Figure 6: Improvement in halitosis outcomes in subjects using K12 Fresh Breath Kit (Group D) compared to standard oral hygiene (Group A). Group D showed significantly greater moderate-to-major improvements in breath quality. Adapted from Jamali et al., 2016.
In addition to S. salivarius K12, there is a related probiotic strain, S. salivarius M18, that is often included in products that aim to support healthy teeth and gums. Human studies show S. salivarius M18 can help against the formation of dental plaque.*14 It has a unique production of enzymes, urease, and dextranase that have been found to slow plaque formation.* It has been found to combat S. mutans and other undesirable oral bacteria linked to periodontal issues.*15 S. salivarius M18 has been shown in studies to support gum health by significantly improving gingival health indicators, including the gingival index and bleeding index during and after use, reducing visible signs of gingivitis, and decreasing inflammation in both children and adults.*16,17 It has been found to help stabilize dental caries progression as well as to reduce the severity and spread of caries in high-risk groups.* In one study, the children who completed a 90-day course of S. salivarius M18 improved their chances of avoiding new caries to 70% compared to the untreated control at 37%.*18 S. salivarius M18 has been shown in studies to demonstrate measurable improvements in oral health after just 4 weeks, with benefits persisting post-intervention.*19
Another probiotic strain, Bifidobacterium animalis subsp lactis (B lactis) HN019, has a growing evidence base indicating that it supports dental health, promotes gum health, promotes a healthy oral microbiota, and supports healthy oral cytokine activity.*20-23
DOSING, SAFETY, and ADMINISTRATION
Dosing of S. salivarius K12 and M18 in most of the clinical trials was 1 billion CFUs in children and up to 5 billion CFUs in adults. Supplementation with S. salivarius K12 seems to offer support to speed the microbial recovery to symbiosis.* Children can safely use it for ongoing support or use it in times of high need (e.g., back to school, cold/flu season, winter, summer camp, etc.). In a safety study, S. salivarius K12 successfully colonized 13/14 individuals after two days of consumption, and there were no adverse reactions.1 A variety of studies, safety and clinical, seem to indicate that once colonized, the S. salivarius K12 seems to maintain for several months, though after stopping the probiotic, gradually the population can reduce over time if not continued, especially if stressors like poor nutrition or a new viral or bacterial illness re-induce dysbiosis (Figure 7). Both S. salivarius K12 and M18 are naturally occurring in humans and are very safe for consumption as a probiotic. S. salivarius K12 has been used for over 20 years with no confirmed reports of adverse effects, with over 1 billion doses of S. salivarius K12 and M18 consumed worldwide. Both strains have been granted GRAS (Generally Recognized as Safe) status by the FDA with no objection. They are both shelf stable and do not require refrigeration, and are safe to take daily.*24
Some consumers who are unfamiliar with the research may raise concerns seeing the name “Streptococcus” on the nutrition facts label due to general public knowledge of S. pyogenes (GAS) as a pathogenic form of strep bacteria. Placebo-controlled trials with S. salivarius K12 have repeatedly confirmed their safety and absence of virulence. When comparing chromosomal DNA of GAS to S. salivarius K12, researchers found that none of the selected GAS virulence factor strains were detected in the S. salivarius K12.*1
Oral probiotics, including S. salivarius K12 and M18, are typically delivered in a dissolvable tablet or lozenge that can be sucked on or chewed one to two times per day. In order to optimize colonization of the oropharynx, it has typically been studied to administer the oral probiotics at bedtime after brushing teeth and to suck on the tablet or lozenge as long as possible, and many of the human clinical studies examine using it daily for a minimum of 90 to 180 days.*
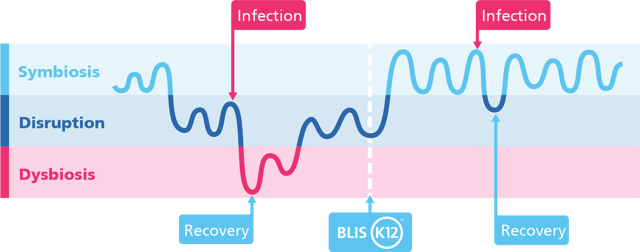
Figure 7: Visual representation of microbial balance across infection and recovery cycles. Without support, repeated infections can lead to periods of dysbiosis and slower microbial recovery. Supplementation with Streptococcus salivarius K12 supports a faster return to symbiosis and may reduce the depth and duration of disruption.* Illustration adapted from Wang et al. 2021.
AUTO-IMMUNITY
Group A beta hemolytic S. pyogenes (GAS) has been associated with a variety of autoimmune diseases such as rheumatic fever, glomerulonephritis, psoriasis, and PANDAS.25 As such, an interesting finding in two more recent studies seems to indicate that S. salivarius K12 supplementation may be beneficial in autoimmune conditions through modulation of the immune system and oral microbiome.* There are four primary ways in which the oral microbiome appears to influence autoimmune diseases: 1) microbial translocation whereby pathogenic bacteria may enter the bloodstream and trigger a strong inflammatory response, 2) molecular mimicry such as the GAS M protein that exhibits structural similarities to cardiac myosin leading to rheumatic heart disease, 3) autoantigen overproduction, and 4) amplification of autoimmunity by cytokines when oral pathogens such as Porphyromonas gingivalis and Fusobacterium nucleatium are overabundant, which can trigger inflammatory cytokines like IL-17 leading to an inflammatory reaction.*26 One hypothesis is that S. salivarius K12 may help autoimmune conditions by helping to maintain the oral microbiome by keeping bacterial pathogens under control.
Researchers have identified dysbiosis in the oral microbiome of humans with Rheumatoid Arthritis (RA) and a deficiency in commensal bacteria such as S. salivarius. In a recent study of mice, they found that supplementation with S. salivarius K12 lowered arthritis scores in both a preventative manner and in a therapeutic manner.*27 The authors hypothesized that interventions like probiotics may be promising to modulate immune dysregulation and alleviate autoimmune symptoms based on previous research that found a modulation of IL-6 and IL-21 receptors.* Further research is needed to confirm if supplementing with S. salivarius K12 can have similar benefits in humans with RA.
Another recent study evaluated S. salivarius K12 in a randomized clinical trial of 198 people suffering from psoriasis.28 The main finding in this study was that 84% of the people taking the S. salivarius K12 lozenges (> 1 billion CFU/day) had at least a 75% improvement in the Psoriasis Area and Severity Index (PASI) score compared to only 42.8% in the control group. The authors concluded that S. salivarius K12 supplementation may be a helpful option for the improvement of psoriasis.*
Given the rising rate of autoimmune disease globally, affecting five to ten percent of the population, and the risk of antibiotic overuse that can disrupt the oral microbiome, future research should seek to identify if S. salivarius K12 may be of use in supporting the immune system for autoimmune diseases related to oral microbiome dysbiosis and GAS.
SUPPORTIVE PROBIOTIC STRAINS
Unlike some products that try to use strains of gut probiotics for the oral microbiome, targeted probiotics from children with healthy microbiomes, like S. salivarius K12 and M18, seem to have more clinical utility. However, some additional strains of probiotics have not been studied as extensively as the K12 and M18 strains that may offer synergistic supportive benefits for the oral microbiome. Lactobacillus rhamnosus and Lactobacillus reuteri have been found to reduce GAS hemolytic activity and inhibit adherence of GAS to host epithelial cells.*29 Lactobacillus paracasei showed inhibition of GAS growth in an in vitro study.*30 L. rhamnosus was found to inhibit GAS biofilm, and survival was reduced significantly.*31 L. paracasei metabolites, including organic acids and H2O2, inhibited GAS.*32 L. rhamnosus and L. reuteri significantly reduced adherence, cytotoxicity, and survivability of GAS in another study by producing lactic acid that degrades the toxic component lipoteichoic acid.*33
SWEETENERS
We were trained as naturopathic physicians to carefully read supplement nutrition labels, including the additional ingredients, when recommending nutritional supplements in clinical practice. I have had the privilege of working with children in my practice for over seventeen years, and also being the proud parent of two little children. One of the key aspects to successful adherence to recommended supplements in pediatrics is making sure the product tastes good and is palatable, especially for very picky eaters and children with intense sensory sensitivities, as is often the case for young children on the autism spectrum. As Mary Poppins said, “A spoonful of sugar helps the medicine go down.” In products designed for children, sweeteners and artificial dyes should always be examined carefully. Some oral probiotics on the market contain sugar-based sweeteners or artificial sweeteners (e.g., sucralose). In order to optimize colonization, oral probiotics are typically recommended to be given at bedtime after brushing teeth. As such, products that are sugar-free using ingredients like isomalt, stevia, or xylitol are preferable for dental health. In addition to its well-documented dental benefits, xylitol may offer additional synergistic benefits to the ENT microbiome as a sugar substitute because it has been found to reduce the adherence of Streptococcus pneumoniae and Haemophilus influenzae to nasopharyngeal cells in vitro and seems to have moderate evidence that prophylactic xylitol use in healthy children attending daycare may reduce the incidence of AOM (acute otitis media).*34
CONCLUSION
The oral microbiome can be disrupted by a variety of environmental factors, which can make a child more susceptible to inflammation, dysbiosis, and recurrent infections. Studies have now identified some key strains of beneficial bacteria that can aid in supporting healthy levels of bacteria in the ears, mouth, and throat when used in daily oral probiotic supplements.* Several key probiotics have been researched to improve the oral microbiome, though a few targeted strains seem to indicate the most promise clinically, especially S. salivarius K12. S. salivarius K12 has a strong safety profile and seems to work via mechanisms to colonize the upper respiratory tract and thereby competitively exclude pathogenic strains of bacteria, provide antimicrobial inhibition, and immune modulation both locally and systemically.* Supporting children’s (and adults’) oral microbiome helps to support their natural immune system defenses, maintain upper respiratory tract health, and support ear, nose, and throat immune health.* The research on this topic is vast, and this article merely supplies an initial overview to scratch the surface of the topic. Future research is needed to further explore potential areas of benefit, such as in autoimmune disease.
Disclaimers:
This article is intended for educational purposes only and does not promote any specific commercial product. The information contained herein is intended for use by licensed medical professionals. Nothing contained in this article should be considered treatment advice or guidance to be used at home without the supervision of a properly trained medical professional. Dr. Oskin owns a nutritional supplement company that produces probiotic supplements. Dr. Oskin has no financial conflicts of interest paid for by the BLIS™ company to report. Images courtesy of BLIS Technologies, 2025.
*These statements have not been evaluated by the U.S. Food & Drug Administration. Products with BLIS™ ingredients and any other probiotic strains and ingredients listed within this article are not intended to diagnose, treat, cure, or prevent any disease.

Dr. Jamie Oskin graduated from Sonoran University of Health Sciences (formerly Southwest College of Naturopathic Medicine) in Tempe, AZ in 2008. After completing a general medicine residency at the Southwest Naturopathic Medical Center, he was accepted into a specialized homœopathy residency sponsored by Standard Homeopathic under Dr. Stephen Messer, ND, DHANP. Dr. Oskin was on the homœopathic faculty at Sonoran University for 9 years and has served on the board of HANP for over a decade. He is always active in the community, publishing well received articles, speaking at conferences, teaching on various topics including the TBR2 method. Dr. Oskin maintains a telehealth practice focused on helping children with developmental disorders. For more information visit https://DrOskin.com/
References
- Burton, J. P., Wescombe, P. A., Moore, C. J., Chilcott, C. N., & Tagg, J. R. (2006). Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Applied and Environmental Microbiology. https://doi.org/10.1128/AEM.72.4.3050- 3053.2006
- Laws, G. L., Hale, J. D. F., & Kemp, R. A. (2021). Human Systemic Immune Response to Ingestion of the Oral Probiotic Streptococcus salivarius BLIS K12. Probiotics and Antimicrobial Proteins. https://doi.org/10.1007/s12602-021-09822-3
- Laws, G. A., Harold, L. K., Tagg, J. R., & Hale, J. D. F. (2022). Interferon Gamma Response in Human Saliva Following Exposure to the Oral Probiotic Streptococcus salivarius BLIS K12. Probiotics and Antimicrobial Proteins. https://doi.org/10.1007/ S12602-022-10010-0
- Bertuccioli, A., Gervasi, M., Annibalini, G., Binato, B., Perroni, F., Rocchi, M. B. L., Sisti, D., & Amatori, S. (2023). Use of Streptococcus salivarius K12 in supporting the mucosal immune function of active young subjects: A randomised double-blind study. Frontiers in Immunology, 14. https://doi.org/10.3389/ fimmu.2023.1129060
- Di Pierro et al., Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children. Drug Healthc Patient Saf. 2014, 13(6):15-20
- Wang, Q., Lin, X., Xiang, X., Liu, W., Fang, Y., Chen, H., Tang, F., Guo, H., Chen, D., Hu, X., Wu, Q., Zhu, B., & Xia, J. (2021). Oropharyngeal Probiotic ENT-K12 Prevents Respiratory Tract Infections Among Frontline Medical Staff Fighting Against COVID-19: A Pilot Study. Frontiers in Bioengineering and Biotechnology, 9. https://doi.org/10.3389/fbioe.2021.646184
- Ilchenko et al. 2019 About prevention of recurrent respiratory diseases in children with microaspiration syndrome. Pediatrics. Eastern Europe 7(4):680-687
- Di Pierro, F., Colombo, M., Zanvit, A., & Rottoli, A. S. (2016). Positive clinical outcomes derived from using Streptococcus salivarius K12 to prevent streptococcal pharyngotonsillitis in children: a pilot investigation. Drug, Healthcare and Patient Safety, 8, 77–81. https://doi.org/10.2147/DHPS.S117214
- Di Pierro, F., & Colombo, M. (2021). The administration of S. salivarius K12 to children may reduce the rate of SARS-CoV-2 infection. In Minerva Medica (Vol. 112, Issue 4, pp. 514–516). Edizioni Minerva Medica. https://doi.org/10.23736/S0026- 4806.21.07487-5
- Guo, H., Xiang, X., Lin, X., Wang, Q., Qin, S., Lu, X., Xu, J., Fang, Y., Liu, Y., Cui, J., & Li, Z. (2022). Oropharyngeal Probiotic ENT-K12 as an Effective Dietary Intervention for Children With Recurrent Respiratory Tract Infections During Cold Season. Frontiers in Nutrition, 9. https://doi.org/10.3389/fnut.2022.900448
- Malcolm, J., Innes-Smith, S., Bennett Bay, M., District, P., Board, H., Moana, T., Pareake O’brien, T., Wright, J., Ball, K., Blunt, T., Hospital, H., & Frampton, C. (2022). Cluster-allocated S. salivarius is more effective than antibiotics-alone reducing pharyngeal Group A Streptococcus prevalence for schoolchildren at risk of Rheumatic fever: a stepped-wedge non-randomized trial.. https://doi.org/10.21203/rs.3.rs-2149210/v1
- Burton, J. P., Chilcott, C. N., Moore, C. J., Speiser, G., & Tagg, J. R. (2006). A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. Journal of Applied Microbiology, 100(4), 754–764. https://doi. org/10.1111/j.1365-2672.2006.02837.x
- Jamali, Z., Aminabadi, N. A., Samiei, M., Deljavan, A. S., Shokravi, M, & Shirazi, S. (2016). Impact of chlorhexidine pretreatment followed by probiotic streptococcus salivarius strain K12 on halitosis in children: A randomized controlled clinical trial. Oral Health and Preventive Dentistry, 14(4), 305–313. https://doi.org/10.3290/j.ohpd.a36521
- Burton, J. P., Drummond, B. K., Chilcott, C. N., Tagg, J. R., Thomson, W. M., Hale, J. D. F., & Wescombe, P. A. (2013). Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: A randomized double-blind, placebo-controlled trial. Journal of Medical Microbiology. https://doi.org/10.1099/jmm.0.056663-0
- Salim, H. P., Mallikarjun, S. B., Raju, S., & Surendranath, A. R. (2023). Randomized Clinical Trial of Oral Probiotic Streptococcus salivarius M18 on Salivary Streptococcus mutans in Preprimary Children. International Journal of Clinical Pediatric Dentistry, 16(2), 259–263. https://doi.org/10.5005/jp-journals-10005-2527
- Scariya, L., Nagarathna, D. v, & Varghese, M. (2015). Probiotics in periodontal therapy. International Journal of Pharma and Bio Sciences, 6(1), P242–P250.
- Kiselnikova, L. P., & Toma, E. I. (2022). Changes in the main dental parameters of preschoolers with caries affected by long-term probiotic intake. Pediatric Dentistry and Dental Prophylaxis, 22(2), 97–102. https://doi.org/10.33925/1683- 3031-2022-22-2-97-102
- Di Pierro, F., Zanvit, A., Nobili, P., Risso, P., & Fornaini, C. (2015). Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clinical, Cosmetic and Investigational Dentistry, 7, 107–113. https://doi.org/10.2147/CCIDE.S93066
- Benic, G. Z., Farella, M., Morgan, X. C., Viswam, J., Heng, N. C., Cannon, R. D., & Mei, L. (2019). Oral probiotics reduce halitosis in patients wearing orthodontic braces: a randomized, triple-blind, placebo-controlled trial. Journal of Breath Research, 13(3), 36010. https://doi.org/10.1088/1752-7163/ AB1C81
- Araujo LDC, Furlaneto FAC, da Silva LAB, et al. Use of the Probiotic Bifidobacterium animalis subsp. lactis HN019 in Oral Diseases. Int J Mol Sci. 2022;23(16):9334.doi:10.3390/ijms23169334
- Invernici MM, Salvador SL, Silva PHF, et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J Clin Periodontol.2018;45(10):1198-1210. doi:10.1111/jcpe.12995
- de Almeida Silva Levi YL, Ribeiro MC, Silva PHF, et al. Effects of oral administration of Bifidobacterium animalis subs. lactis HN019 on the treatment of plaque-induced generalized gingivitis. Clin Oral Investig. 2023;27(1):387-398. doi:10.1007/s00784-022-04744-y
- Invernici MM, Furlaneto FAC, Salvador SL, et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS One. 2020;15(9):e0238425. doi:10.1371/journal.pone.0238425
- Burton, J. P., Cowley, S., Simon, R. R., McKinney, J., Wescombe, P. A., & Tagg, J. R. (2011). Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 49(9), 2356–2364. https://doi.org/10.1016/j.fct.2011.06.038
- Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155(2):264-271.
- Huang X, Huang X, Huang Y, et al (2023) The oral microbiome in autoimmune diseases: friend or foe? Journal of Translational Medicine 2023 21:1 21:1–24. https://doi.org/10.1186/S12967-023-03995-X
- Li J, Li S, Jin J, et al (2024) The aberrant tonsillar microbiota modulates autoimmune responses in rheumatoid arthritis. JCI Insight. https://doi.org/10.1172/jci.insight.175916
- Zangrilli A, Diluvio L, Di Stadio A, Di Girolamo S (2022) Improvement of Psoriasis Using Oral Probiotic Streptococcus salivarius K-12: a Case-Control 24-Month Longitudinal Study. Probiotics Antimicrob Proteins 14:573–578. https://doi.org/10.1007/s12602-022-09937-1
- Saroj SD, Maudsdotter L, Tavares R, Jonsson AB. Lactobacilli Interfere with Streptococcus pyogenes Hemolytic Activity and Adherence to Host Epithelial Cells. Front Microbiol. 2016 Jul 29;7:1176. doi: 10.3389/fmicb.2016.01176. PMID: 27524981; PMCID: PMC4965460.
- Brandi J, Cheri S, Manfredi M, Di Carlo C, Vita Vanella V, Federici F, Bombiero E, Bazaj A, Rizzi E, Manna L, Cornaglia G, Marini U, Valenti MT, Marengo E, Cecconi D. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci Rep. 2020 Jul 14;10(1):11572. doi: 10.1038/s41598-020-68483-4. PMID: 32665600; PMCID: PMC7360600
- Gómez-Mejia A, Orlietti M, Tarnutzer A, Mairpady Shambat S, Zinkernagel AS.2024.Inhibition of Streptococcus pyogenes biofilm by Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus. mSphere9:e00430-24.https://doi.org/10.1128/msphere.00430-24
- SU Ben-Xian, AI Jing, Zhu De-Quan, MENG Xiang-Chen. Inhibition of Streptococcus pyogenes by lactobacilli metabolites[J]. Microbiology China, 2015, 42(1): 117-124 (Chinese Language)
- Maudsdotter L, Jonsson H, Roos S, Jonsson AB. Lactobacilli reduce cell cytotoxicity caused by Streptococcus pyogenes by producing lactic acid that degrades the toxic component lipoteichoic acid. Antimicrob Agents Chemother. 2011 Apr;55(4):1622-8. doi: 10.1128/AAC.00770-10. Epub 2011 Jan 18. PMID: 21245448; PMCID: PMC3067128.
- Azarpazhooh A, Lawrence HP, Shah PS. Xylitol for preventing acute otitis media in children up to 12 years of age. Cochrane Database Syst Rev. 2016 Aug 3;2016(8):CD007095. doi: 10.1002/14651858.CD007095.pub3. PMID: 27486835; PMCID: PMC8485974.