Ralph Esposito, ND, LAc
Naturopathic Perspective
Sarcopenia: Poverty of the Flesh
Ask some young adults in the prime of their 20s about their perception of the elderly, and you may find they most often define aging as “getting old and weak.” Sarcopenia – poverty of the flesh – is a condition of aging. Our society views sarcopenia as a natural process, as it is unofficially defined as the age-related loss of skeletal muscle mass and function. There is currently no universally accepted definition of sarcopenia, and since the term was coined in 1989, the medical field still debates about which parameters best define sarcopenia.
It is estimated that by the eighth decade of life, men and women have lost approximately 50% of their muscle mass,1 with estimates of approximately 0.8% of annual muscle loss2 beginning as early as 30 years old in inactive adults.3 Therefore, it is imperative for practitioners to understand that sarcopenia is not necessarily a disease of old age but rather a disease of old aging, as it is a slow process that begins in early life.
To be clear, sarcopenia must be distinguished from cachexia and wasting, whereby cachexia is involuntary severe loss of weight, muscle, and fat due to an underlying disease, and wasting is involuntary weight loss due to inadequate nutrient intake. In attempts to improve the diagnostic accuracy of sarcopenia, several measures have been agreed upon, including muscle strength, muscle mass (via BIA or DEXA) and functional measurements related to grip strength and walking speed.4
Yet from a naturopathic perspective, it is not necessarily considered acceptable or normal to lose muscle mass as part of the act of aging. Regardless of the definition of sarcopenia, it is essential that we understand the cause of muscle loss, muscle weakness, and functional losses. A precision medicine approach does not simply provide anti-sarcopenia therapy, but instead addresses the foundations of muscle loss. Precision medicine utilizes molecular and systems biology to dig deep into networks and pathways while incorporating biochemical individuality to treat aberrant function.
Mitochondrial Dysfunction & Inflammation
If we are to dig deep into uncovering the dysfunction occurring on the cellular level in the muscle-aging process, then it seems appropriate to begin with analyzing the ATP powerhouse of the cell, mitochondria. Mitochondria are foundational in energy production, apoptosis, and redox reactions, and, as such, it should be expected that mitochondrial dysfunction would play a critical role in chronic muscle atrophy, or sarcopenia.
Reactive oxygen species (ROS), bioenergetic failure, and apoptosis induction are the 3 major routes for how mitochondrial dysfunction progresses to muscle loss. These 3 factors can be grounded in aberrant autophagy, a cellular housekeeping mechanism whereby organelles and cells undergo a type of cell clean-up when function has been lost to improve survival and function.5 Autophagy inhibition is thought to contribute to sarcopenia by not only inhibiting a stressed myocyte from being modified or simply destroyed, but also by inhibiting myokine secretions that prevent a cellular stress response; the result is a myofiber that is alive and active but atrophic and useless.6 Muscle atrophy on a molecular level can be attributed to chronically increased ROS, oxidative damage, damaged proteins, protein misfolding, and dysfunctional organelles. Together, these factors interrupt the function of the muscle cells, interrupting autophagy, inducing apoptosis, and leading to atrophy, which is seen macroscopically in the aging muscle.7
Inflammation, more specifically tissue levels of inflammatory cytokines and markers such as IL-6 and nuclear factor-kappa B (NF-kβ) are increased in elderly individuals with poor physical performance,8 which further supports the idea of oxidative stress being a possible contributor to muscle mass decline in the elderly.9 Tumor necrosis factor-alpha (TNFα) and C-reactive protein (CRP) are 2 additional inflammatory markers associated with decreased physical performance, gait speed, and muscle mass in the geriatric population.10,11 These inflammatory markers are involved in the induction of apoptosis, suggesting that these markers are not only indicators of inflammation but directly induce myocyte destruction.12
Hormones
Avoid catabolism and promote anabolism – 2 rules in preserving and building muscle in the elderly. Insulin, one of the body’s multiple anabolic hormones, is a prime target in sarcopenia because of its ability to induce muscle protein synthesis. However, this becomes an issue in the elderly primarily because insulin resistance and metabolic syndrome is highly prevalent.13 Furthermore, non-diabetic elderly men and women are resistant to the anabolic actions of insulin.13 Contrary to expectations, muscle protein synthesis is equal in both young and old adults,14 which begs the question of why sarcopenia occurs in the elderly if their muscle protein synthesis is unchanged. If muscle protein synthesis unlikely accounts for the chronic loss of muscle during aging, then the elderly likely exhibit anabolic resistance to insulin and possibly other hormones.13,15
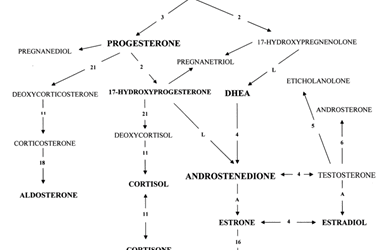
Low levels of testosterone (T), one of the most prominent anabolic hormones in men and women, have been associated with decreased muscle mass and strength and physical performance.16 Testosterone supplementation in elderly men has been shown to improve these parameters. It is imperative to optimize endogenous testosterone in preventing sarcopenia.16 Dehydroepiandrosterone (DHEA), a precursor to testosterone, is unique in that skeletal muscle can directly convert DHEA into T. One would expect this to result in increased muscle mass and strength. However, a large systematic review analyzing DHEA supplementation in older adults found inconsistent results for muscle strength and physical performance.17
The major female hormone, estrogen, functions to activate satellite cells, which in turn improves muscle strength and muscle recovery. Postmenopausal women with low estrogen levels experience declines in muscle mass and strength.18,19
Biomarkers
Of course, any physician could order an extensive amount of lab work and come to many conclusions based on these numbers, but the truth is that there is no 1 single biological marker of physical frailty and sarcopenia. Understanding the functional aspect of sarcopenia from a holistic perspective calls for a multi-dimensional approach in assessing the complex pathophysiology of sarcopenia.
Several biomarkers and analytics can allow the physician to better understand the multifactorial causes of sarcopenia:
- Bioelectrical-impedance analysis (BIA): Utilizing this tool to assess changes in body fat and lean muscle mass allows objective analysis of sarcopenia progression and improvement
- CRP and IL-6: These 2 inflammation markers have been positively correlated with decreased muscle strength and physical performance11,20
- HbA1c, fasting glucose and insulin: The prevalence of insulin resistance in the elderly and the impact of advanced glycation end-products (AGEs) on mitochondrial function and inflammation warrants close monitoring of glucose levels. Furthermore, high insulin levels may often precede elevated HbA1c, allowing earlier preventive intervention.
- 8-Hydroxy-2’-deoxyguanosine (8-OHdG): This is a unique urinary marker associated with mitochondrial oxidative damage, which can be useful in not only identifying current oxidative damage but also sarcopenia response to treatment; evidence suggests improvements in 8-OHdG from resistance training in older adults21
- 25-hydroxyvitamin D3: Given the physiological role of vitamin D in muscle growth and development, it comes as no surprise that low levels (<30 ng/L) have been correlated with sarcopenia.22 Supplementation to achieve adequate levels of vitamin D3 is appropriate in older adults.
Therapeutic Approach
Exercise as a therapeutic in preventing and treating sarcopenia is a foregone conclusion – we know exercise induces muscle growth. But in precision medicine, the goal is why, how much, how often, and what type. Simply advising an inexperienced older adult to exercise is only marginally effective unless designed precisely. Resistance training with proper intensity, volume, and velocity combined equally with endurance exercise will improve muscle strength and mass and reduce risk of injury during training. Intensity >70% of 1-rep max, volume >3 sets per exercise, and bursts of movement with high velocity have been found to be most effective for improving muscle strength, function, and neuromuscular changes in older adults.23-25
Often overlooked is the neuromuscular component of exercise. Balance exercises can train the central nervous system to coordinate with the musculoskeletal system to induce the proper neuromuscular stimulus to activate muscle growth and functionality.25 Satellite cells, or muscle resident stem cells, which are responsible for muscle hypertrophy, decrease with aging and sarcopenia. Resistance training taps into satellite cells that hold the nuclear reserve to enable muscle regeneration; this in turn results in improvements in muscle function, strength, and mass – essentially the foundation for reversing sarcopenia.
We know that protein alone can stimulate muscle protein synthesis in the young; however, this ability is blunted in the elderly.26 Adequate high-quality and properly-dosed protein works synergistically with resistance training to stimulate an anabolic response, ie, the induction of muscle growth and strength.2 Yet, if we are to be precise, the questions become how much, what kind, and how often? The elderly require more protein intake overall than their younger counterparts in order to induce muscle growth and anabolism – an amount estimated to be 1.2 g/kg/d. However, the type of protein is important because the essential amino acids (EAA), in particular leucine, trigger the regulatory proteins that initiate muscle protein synthesis. The magic dose for stimulating muscle growth appears to be 25-30 g (>10g of EAA) of protein, including 2.5 g of leucine, TID.2 Furthermore, a metabolite of leucine, beta-hydroxy-beta-methylbutyrate (ß-HMB), has been shown to induce muscle growth, inhibit muscle breakdown, and improve physical performance in older adults.26,27 We must also pay mind to digestive function of older adults, as the elderly are often less capable of digesting and assimilating high-dose protein meals, which makes digestive enzymes a prudent therapeutic in many cases.
Addressing mitochondrial health is foundational in treating sarcopenia. Coenzyme Q-10 (CoQ10) supplementation provides the mitochondria with adequate energy via its role in the electron transport chain. But more importantly, low CoQ10 may be a risk factor in sarcopenia.28 From a functional perspective, CoQ10 can be utilized to improve mitochondrial health, which not only improves ATP production but may also reduce mitochondrial oxidative damage and apoptosis. There is minimal risk associated with CoQ10, and supplementation with 200 mg daily may induce improvements.
Conclusion
If the healthcare society is unable to concur on a definition and clear cause for sarcopenia, then it is up to practitioners to take a Precision Medicine approach, tailoring the treatments and assessment on a molecular and systems-biology level. This is necessary given the heterogeneity and complexity of sarcopenia’s pathophysiology. In light of such complexity, practitioners must employ requisite variety in the treatment strategy. This means that if we wish to properly address the diversity of issues in sarcopenia, our repertoire of therapeutics must be just as diverse and flexible as the pathophysiology of sarcopenia itself. Muscle strength, function, and mass is not lost by 1 singular factor; it is submerged in a network of dysfunction. Hence, to address the cause we must tap into the network utilizing multiple different approaches and pathways to revitalize form and functionality.
[Refs]:
- Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52(5):B267-B276.
- Phillips SM. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv Nutr An Int Rev J. 2015;6(4):452-460.
- Nair KS. Muscle protein turnover: methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:107-112.
- Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) ”cachexia-anorexia in chronic wasting diseases” and ”nutrition in geriatrics.” Clin Nutr. 2010;29(2):154-159.
- Teixeira Vde O, Filippin LI, Xavier RM. Mechanisms of muscle wasting in sarcopenia. Rev Bras Reumatol. 2012;52(2):252-259.
- Jiao J, Demontis F. Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr Opin Pharmacol. 2017;34:1-6.
- Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394(3):393-414.
- Buford TW, Cooke MB, Manini TM, et al. Effects of Age and Sedentary Lifestyle on Skeletal Muscle NF- B Signaling in Men. J Gerontol A Biol Sci Med Sci. 2010;65(5):532-537.
- Marzetti E, Lees HA, Manini TM, et al. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS One. 2012;7(2):e32829.
- Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326-M332.
- Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242-248.
- Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11(5):801-809.
- Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20(6):768-769.
- Mucci M, Carraro C, Mancino P, et al. Soy isoflavones, lactobacilli, Magnolia bark extract, vitamin D3 and calcium. Controlled clinical study in menopause. Minerva Ginecol. 2006;58(4):323-334.
- Fry CS, Rasmussen BB. Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci. 2011;4(3):260-268.
- Vitale G, Cesari M, Mari D. Aging of the endocrine system and its potential impact on sarcopenia. Eur J Intern Med. 2016;35:10-15.
- Baker WL, Karan S, Kenny AM. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc. 2011;59(6):997-1002.
- Messier V, Rabasa-Lhoret R, Barbat-Artigas S, et al. Menopause and sarcopenia: A potential role for sex hormones. Maturitas. 2011;68(4):331-336.
- Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol Ser A Biol Sci Med Sci. 2012;67(11):1140-1152.
- Tiainen K, Hurme M, Hervonen A, et al. Inflammatory markers and physical performance among nonagenarians. J Gerontol Ser A Biol Sci Med Sci. 2010;65(6):658-663.
- Parise G, Brose A, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol. 2005;40(3):173-180.
- De Spiegeleer A, Petrovic M, Boeckxstaens P, Van Den Noortgate N. Treating sarcopenia in clinical practice: where are we now? Acta Clin Belg. 2016;71(4):197-205.
- Izquierdo M, Ibañez J, HAkkinen K, et al. Once weekly combined resistance and cardiovascular training in healthy older men. Med Sci Sports Exerc. 2004;36(3):435-443.
- Valenzuela T. Efficacy of progressive resistance training interventions in older adults in nursing homes: a systematic review. J Am Med Dir Assoc. 2012;13(5):418-428.
- Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008;18(6):773-782.
- Moore DR, Churchward-Venne TA, Witard O, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol Ser A Biol Sci Med Sci. 2015;70(1):57-62.
- Flakoll P, Sharp R, Baier S, et al. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition. 2004;20(5):445-451.
- Fischer A, Onur S, Niklowitz P, et al. Coenzyme Q10 Status as a Determinant of Muscular Strength in Two Independent Cohorts. PLoS One. 2016;11(12):e0167124.
Image Copyright: <a href=’https://www.123rf.com/profile_stylephotographs’>stylephotographs / 123RF Stock Photo</a>
 Ralph Esposito, ND, LAc, is a naturopathic physician and licensed acupuncturist specializing in urology and men’s health. He has been published and is a peer reviewer in well-respected medical journals. Further, Dr Esposito has authored several medical textbook chapters and has designed education modules for health professionals specifically on male and female endocrine dysfunction, urological conditions, andropause, exercise, fitness, and men’s health. He currently practices at the Center of Excellence in Generative Medicine and collaborates in research at the NYU Integrative and Functional Urology Center. Dr Esposito also holds a position as adjunct professor at NYU, where he lectures on integrative medicine.
Ralph Esposito, ND, LAc, is a naturopathic physician and licensed acupuncturist specializing in urology and men’s health. He has been published and is a peer reviewer in well-respected medical journals. Further, Dr Esposito has authored several medical textbook chapters and has designed education modules for health professionals specifically on male and female endocrine dysfunction, urological conditions, andropause, exercise, fitness, and men’s health. He currently practices at the Center of Excellence in Generative Medicine and collaborates in research at the NYU Integrative and Functional Urology Center. Dr Esposito also holds a position as adjunct professor at NYU, where he lectures on integrative medicine.