A Promising Cancer Therapy
Student Scholarship – 2nd Place Research Review
Erin Rurak, NMS
Gurdev Parmar, ND, FABNO
Fever is an evolutionary-conserved immune system response to infection that is present throughout the animal kingdom. This suggests that fever confers a survival advantage. In the practice of medicine, elevated body temperature has been used to combat illnesses ranging from systemic bacterial infection to malignancy.1 Reports from as early as the 1700s note that occurrence of infectious diseases and resultant fevers could lead to spontaneous regression of malignancies.1,2 After observing that accidental erysipelas (Streptococcus pyogenes) infections in hospitalized patients inhibited tumor growth, 2 German physicians, W. Busch (in 1868) and F. Fehleisen (in 1882), intentionally induced infections and reported tumor regression.1,3An American surgeon, William Coley, reported similar outcomes in the 1890s and went on to create “Coley’s toxin,” a mixture of heat-killed, fever-inducing bacteria, which were sold in the United States for the treatment of cancer until 1963.3 This drug therapy showed promising results for a number of cancers, including sarcomas, lymphomas, and melanomas.1 Interestingly, Coley found a positive correlation between the chance of recovery and the intensity of fever and strength of the induced immune response.1
Coley’s toxin was replaced with more modern cytotoxic cancer therapies, including radiation and chemotherapy.3 These treatments combined with surgery have been the mainstay in modern cancer treatment for decades. However, conventional cancer treatments are effective in only about half of cancer patients.3 As a result, alternative therapies in cancer treatment are urgently needed.
Hyperthermia
Coley’s results suggest that the immune system, once stimulated, can play a role in tumor treatment.1 Hyperthermia treatments induced by physical methods rather than pharmacological means have replaced Coley’s toxin as a means to enlist immune forces and to directly treat cancer.4 In hyperthermia therapy, heat is applied either alone to induce tumor cell death, or as an adjunct to other cancer treatments.5 Over 40 clinical studies have demonstrated the benefit of hyperthermia.2,5 These positive results spurred the development of a variety of hyperthermia devices to produce local, regional, or whole-body hyperthermia.
Local Regional Hyperthermia
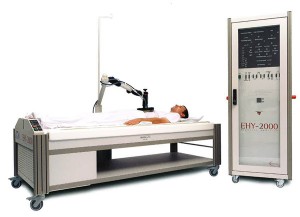
In local hyperthermia, heat is applied to an area where a solid tumor is located (Figure 1). There are several approaches that can be chosen, based on the location of the tumor. External techniques use heat generated by an external source.6,7 Internal hyperthermia uses a thin needle or probe for placement directly into the tumor site where heat is released in and around the tumor site. Treatment areas are heated to 104-113 °F.7 This specific temperature range promotes selective heat-induced tumor cell death, as well synergistic effects when used in combination with radiotherapy and chemotherapy.5,8
Figure 1
Regional Hyperthermia
In regional hyperthermia, a part of the body such as a limb or body cavity is heated. As with local hyperthermia, target temperature ranges are 104-113 °F.6,7 In one approach, known as regional perfusion, the blood is removed, heated and returned to blood vessels that supply the targeted region. Frequently, chemotherapeutics are infused at the same time.6 The goal is to weaken malignant cells, making them more susceptible to chemotherapy or radiation.8,9
Fever-Range, Whole Body Hyperthermia
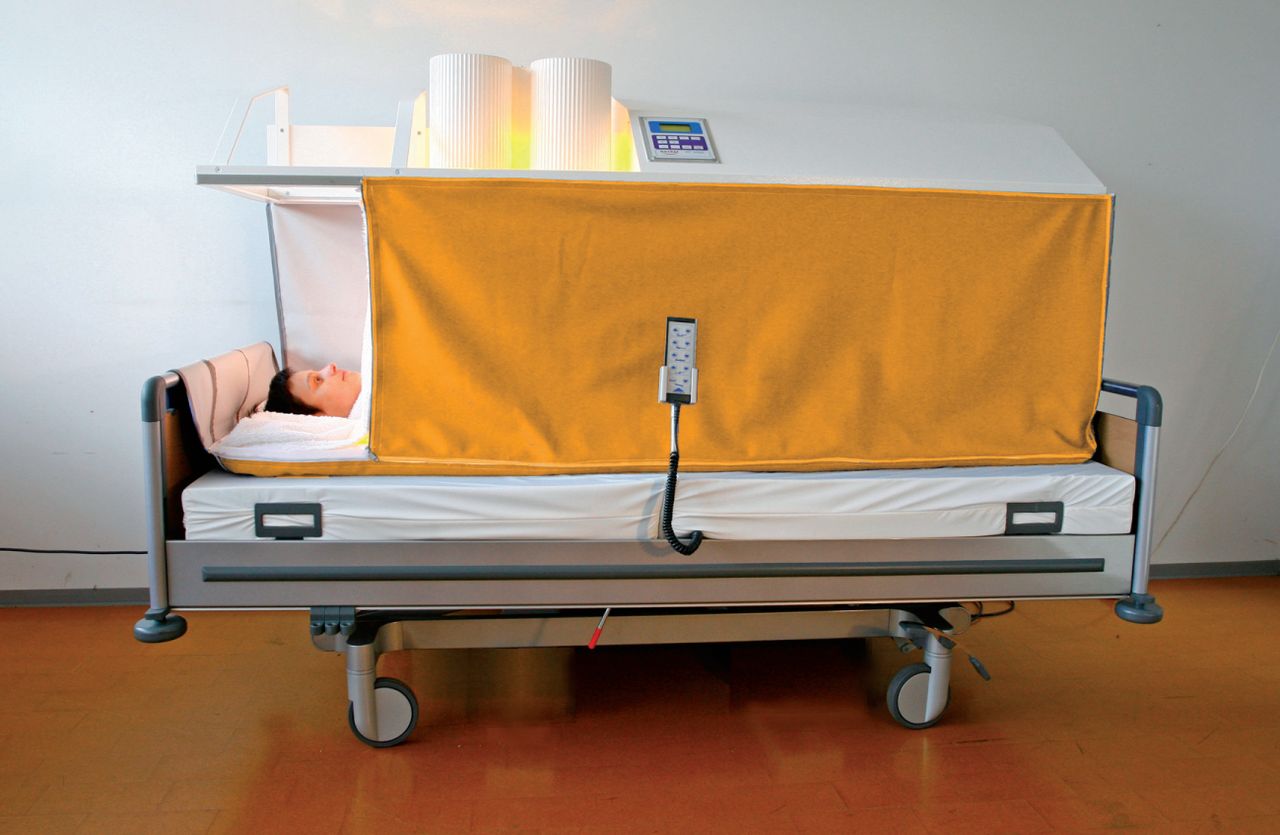
In whole body hyperthermia, heat is applied to the whole body, excluding the head, to elevate core body temperature into fever range (101.3-104 °F)8 (Figure 2). Several methods are presently used; in all, energy is introduced into the body while energy losses are minimized.5,8 This treatment is used primarily for metastatic cancers where there has been distant spread of cancer cells from the primary tumor site.8 Treatments last for up to 6 hours and, as a result, specialized equipment and monitoring are required.
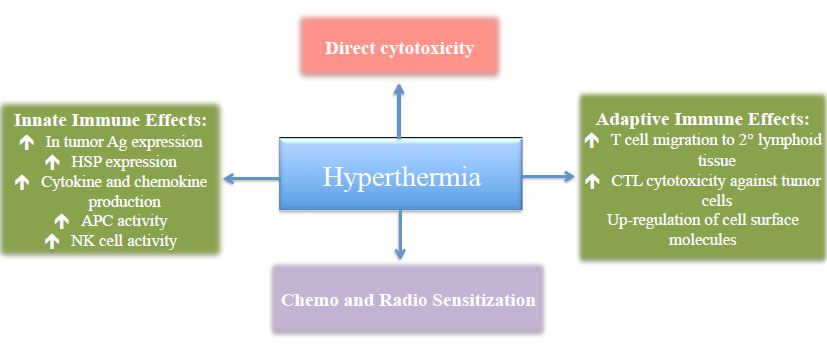
Regardless of which method is utilized to induce hyperthermia, the action is the same. Heat preferentially kills malignant cells through complex biological reactions including direct cytotoxic effects, immunostimulation, and synergistic effects with other cancer treatments (Figure 3).
Figure 2
Figure 3. Mechanisms of Action of Hyperthermia in Tumor Cell Control
HSP – Heat shock proteins; APC – Antigen-presenting cell; NK cell – Natural killer cell; CTL – Cytotoxic T lymphocytes
Hyperthermia Mechanisms of Action
Hyperthermia and Tumor Selectivity
Malignant cells are more sensitive to high temperature compared to healthy tissue.9 This selectivity toward cancer cells relies on differences between normal and tumor cell physiology.5,9 Tumor architecture and vasculature supply is more chaotic than in healthy tissue.10 Tumors vigorously build vascular networks. This vasculature formation is indiscriminate, leading to the production of an immature, structurally-defective microvascular system that is less resilient to perfusion shifts.5,8,10 This creates adverse tumor micro-environmental conditions, including hypoxia, acidosis, and energy depletion.11 This leaves the tumor cells more sensitive to hyperthermia.5,10
Cancer cells exhibit differences in their cell morphology, membrane fluidity, and gene expression.9 Heat increases membrane fluidity and instability of cancer cells, leading to cell death directly, or indirectly through increased delivery of cytostatic chemotherapy agents.9 Furthermore, heat shock induces expression of p53, a tumor suppressor transcription factor that is often mutated or decreased in cancer cells.9,12 Hyperthermia thus has direct cytotoxic effects that can lead to an immediate reduction in tumor size.
Hyperthermia and Anti-Tumor Immunity
Hyperthermia minimizes local and distal tumor recurrence by inducing protective anti-tumor immune responses.2,13 In order to initiate an immune response against malignant cells, antigen-presenting cells (APCs) must take up and process tumor peptides for presentation to naïve T cells in draining lymph nodes.11,13 Lymphocytes will mature and proliferate into cytotoxic T lymphocytes (CTLs) and CD4+ helper cells, mounting an adaptive immune response against tumor cells after binding to tumor antigens on APCs.11
Fever range hyperthermia (101.3-104 °F) affects many branches of the innate and adaptive immune system, contributing to long-term tumor growth inhibition.2,4 Hyperthermia upregulates tumor antigen expression and increases APC activity.11 Heat exposure directly increases antigen expression and release from tumor cells.13 Furthermore, hyperthermia triggers release of heat shock proteins (HSP),4,11 which present tumor antigens to APCs, thereby increasing tumor recognition.11
Heat induces secretion of chemokines and cytokines within tumor micro-environments.2,11 These chemicals draw APCs into tumors and increase macrophage activity and tumor regression.2 Increased tumor antigen and HSP expression, coupled with enhanced activity and infiltration of APCs into tumor sites, heightens tumor recognition by activating many branches of the innate immune system.
Activation of the adaptive immune system favors tumor regression and long-term protection against tumor growth.11 For lymphocytes to become activated to recognize and destroy malignant cells, they must first migrate out of the blood and into the secondary lymphoid tissue and contact APC-presenting tumor antigen complexes.13 Fever-range hyperthermia upregulates adhesion molecules present on endothelial venules, allowing more lymphocytes to enter secondary lymphoid tissue where an adaptive immune response will be initiated.2 Exposure to heat also upregulates cell markers such as Fas ligand, influencing the cytolytic capacity of CTLs.13 These effector T cells travel to distant sites, where they play a role in long-term anti-tumor immunity.
The recognition of target cells by effector T cells, specifically CTLs, requires that target cells express major histocompatibility complexes (MHC) class I antigens.11,13 Tumor cells downregulate MHC-I to escape recognition by the immune system.11 MHC-I loss renders tumor cells less susceptible to T cell-mediated immunity. Because natural killer (NK) cells target MHC-deficient cells, they are essential in countering this evasive strategy.11,13 Hyperthermia increases NK cell activity and, thus, protective anti-tumor cell surveillance.4,11,13
Clinical Results of Hyperthermia
Evidence for the use of hyperthermia in cancer care continues to grow.4,5,12 The use of hyperthermia alone has shown improvements in both partial and complete response rates for several malignancies, including squamous cell cancers, adenocarcinomas, and melanomas.14,15 Hyperthermia, however, has shown most benefit when included in a multimodal treatment approach.
The addition of locoregional hyperthermia during chemotherapy improves drug delivery to tumor cells and counteracts drug resistance by cisplatin, doxorubicin and mitomycin C.5 Studies report improved response rates in bladder, lung, esophageal, cervical, and ovarian cancer.5,16,17 Cervical cancer had a 50% response rate with combination therapy, compared to an expected 15% response with chemotherapy alone.5 Hyperthermia and chemotherapy have produced positive outcomes in pediatric populations with unresectable localized germ-cell tumors, as well as sarcomas refractory to standard therapy.18 Wessalowski et al reported that combination treatment was well tolerated and led to a local tumor response in 77% of pediatric patients.18
Hyperthermia improves radiation effects by increasing tissue oxygenation while inhibiting the repair of radiation-induced damage.5 Cancers showing significantly better response to combination therapy include metastatic lymph nodes of head and neck tumors, melanoma, breast cancer, glioblastoma multiforme, bladder cancer, cervical cancer, and esophageal cancer.5,19 Valdagni et al examined the effects of combined radiation therapy on patients with stage IV squamous cell cancer of the head and neck with metastatic cervical lymph nodes.20 The complete response rate improved from 41% to 83%, with a 5-year local control increasing from 24% to 69%, without increasing toxicity.20 Inclusion of hyperthermia in cancer treatment may improve response rates, with minimal risk for adverse reactions.
When William Coley created his bacterial concoction more than a century ago, the mechanisms by which they caused tumor regression were unknown. Coley assumed that it was the body’s innate ability to mount an immune defense at work. Coley’s belief that fever and heat were fundamental to immune defense sounds all too familiar to naturopathic physicians. We might be tempted to use the term Vis Medicatrix Naturae to explain the benefit of heat and infection.
Given how many cancers remain resistant to modern treatments, especially when used alone, hyperthermia’s ability to enhance tumor regression through its unique ability to increase anti-tumor immunity deserves our attention. The promise of better outcomes for our patients by adding hyperthermia to standard cancer treatments is enormously alluring.
 Erin Rurak, NMS is a 4th year student at the Boucher Institute of Naturopathic Medicine (BINM). She has a number of interests, including integrative oncology, geriatrics, and physical medicine. She has a strong passion for clinical research and hopes to integrate this within her practice after graduation. Prior to enrolling at BINM, Erin spent 2 years at British Columbia’s Children’s Hospital taking part in research related to childhood obesity. She evaluated the efficacy of family-based weight intervention programs within BC’s Lower Mainland, as well as in remote First Nations communities in Northern BC. She developed a strong interest in preventative and holistic medicine throughout these 2 years. She attended the University of British Columbia, receiving an undergraduate degree in microbiology and immunology, and a minor in art history. On a personal note, Erin enjoys spending time with her family and many pets. Her favorite activity is to spend time on the farm with her horses and long-haired miniature dachshund, Karma.
Erin Rurak, NMS is a 4th year student at the Boucher Institute of Naturopathic Medicine (BINM). She has a number of interests, including integrative oncology, geriatrics, and physical medicine. She has a strong passion for clinical research and hopes to integrate this within her practice after graduation. Prior to enrolling at BINM, Erin spent 2 years at British Columbia’s Children’s Hospital taking part in research related to childhood obesity. She evaluated the efficacy of family-based weight intervention programs within BC’s Lower Mainland, as well as in remote First Nations communities in Northern BC. She developed a strong interest in preventative and holistic medicine throughout these 2 years. She attended the University of British Columbia, receiving an undergraduate degree in microbiology and immunology, and a minor in art history. On a personal note, Erin enjoys spending time with her family and many pets. Her favorite activity is to spend time on the farm with her horses and long-haired miniature dachshund, Karma.
Gurdev Parmar, ND, FABNO was the first Canadian naturopathic physician to qualify for a fellowship with the American Board of Naturopathic Oncology in 2007, furthering his focus in the preention and treatment of cancer. He and his wife, Dr Karen Parmar, launched the Integrated Health Clinic in 2000 and have since facilitated its growth to become one of the largest and most successful integrated healthcare facilities in Canada. Dr Parmar was a consulting naturopathic physician at the Lions Gate Hospital cancer clinic from 2008 to 2012. He has established a collaborative relationship with many oncologists, nurses, and other practitioners to ensure patients are provided the most current evidence-guided treatments possible. Dr Parmar is an active member of the College of Naturopathic Physicians of BC, as well as a member of the American Society of Clinical Oncology and the Oncology Association of Naturopathic Physicians.
References
1. Bickels J, Kollender Y, Merinsky O, Meller I. Coley’s toxin: Historical perspective. Isr Med Assoc J. 2002;4(6):471-472.
2. Peer AJ, Grimm MJ, Zynda ER, Repasky EA. Diverse immune mechanisms may contribute to the survival benefit seen in cancer patients receiving hyperthermia. Immunol Res. 2010;46(1-3):137-154.
3. Patyar S, Joshi R, Byrav DS, et al. Bacteria in cancer therapy: A novel experimental strategy. J Biomed Sci. 2010;17(1):21.
4. Sulyok I, Fleischmann E, Stift A, et al. Effect of preoperative fever-range whole-body hyperthermia on immunological markers in patients undergoing colorectal cancer surgery. Br J Anaesth. 2012;109(5):754-761.
5. Van der Zee J. Heating the patient: A promising approach? Ann Oncol. 2002;13(8):1173-1184.
6. National Cancer Institute. Hyperthermia in cancer treatment. NCI Web site. http://m.cancer.gov/topics/factsheets/hyperthermia. Updated 2011. Accessed August 7, 2013.
7. American Cancer Society. Statement on hyperthermia. http://www.cancer.org/treatment/treatmentsandsideeffects/treatmenttypes/hyperthermia. Updated 2011. Accessed August 7, 2013.
8. Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33-56.
9. Csoboz B, Balogh GE, Kusz E, et al. Membrane fluidity matters: Hyperthermia from the aspects of lipids and membranes. Int J Hyperthermia. 2013;29(5):491-499.
10. Vaupel PW, Kelleher DK. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int J Hyperthermia. 2010;26(3):211-22.
11. Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528-542.
12. Atanackovic D, Nierhaus A, Neumeier M, et al. 41.8 degrees C whole body hyperthermia as an adjunct to chemotherapy induces prolonged T cell activation in patients with various malignant diseases. Cancer Immunol Immunother. 2002;51(11-12):603-613.
13. Muthana M, Multhoff G, Pockley AG. Tumour infiltrating host cells and their significance for hyperthermia. Int J Hyperthermia. 2010;26(3):247-255.
14. Manning MR, Cetas TC, Miller RC, et al. Clinical hyperthermia: Results of a phase I trial employing hyperthermia alone or in combination with external beam or interstitial radiotherapy. Cancer. 1982;49(2):205-216.
15. Gabriele P, Orecchia R, Ragona R, et al. Hyperthermia alone in the treatment of recurrences of malignant tumors. experience with 60 lesions. Cancer. 1990;66(10):2191-2195.
16. Colombo R, Da Pozzo LF, Lev A, et al. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy versus chemotherapy alone for superficial bladder cancer. J Urol. 1996;155(4):1227-1232.
17. Engelhardt R. Summary of recent clinical experience in whole-body hyperthermia combined with chemotherapy. Recent Results Cancer Res. 1988;107:200-204.
18. Wessalowski R, Schneider DT, Mils O, et al. An approach for cure: PEI-chemotherapy and regional deep hyperthermia in children and adolescents with unresectable malignant tumors. Klin Padiatr. 2003;215(6):303-309.
19. Van der Zee J, Treurniet-Donker AD, The SK, et al. Low dose reirradiation in combination with hyperthermia: A palliative treatment for patients with breast cancer recurring in previously irradiated areas. Int J Radiat Oncol Biol Phys. 1988;15(6):1407-1413.
20. Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys. 1994;28(1):163-169.