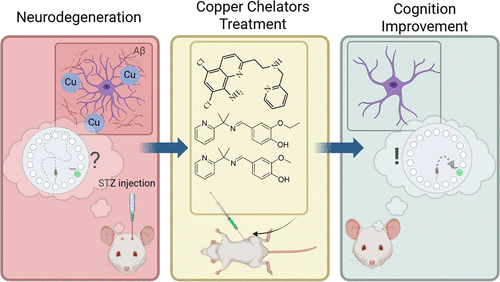
A new compound reduced hippocampal inflammation and improved memory in Alzheimer’s model rats
A research team in Brazil has published animal data showing a copper chelating compound can cross the blood brain barrier, pull copper out of beta amyloid plaques, and reverse memory deficits in rats with induced Alzheimer’s disease. The study appeared in ACS Chemical Neuroscience in August 2025.
Practitioners who test copper levels in patients with cognitive decline now have mechanism data to go with clinical intuition. The study shows what happens when you actually remove the copper.
What They Did
Professor Giselle Cerchiaro’s team at the Federal University of ABC synthesized nine compounds. They screened them first with computer modeling, then cell culture, then animal testing. Three passed the early filters: L09, L10, and L11. All three could bind copper and pull it away from beta amyloid in test tube experiments.
L10 worked best in the animals.
Rats received streptozotocin injections to induce an Alzheimer’s like state, then got L10 at 12.5 mg/kg daily for five days. When the researchers looked at hippocampal tissue, they found lower astrocyte activation (measured by GFAP), reduced beta amyloid, and normalized levels of ATP7B, a copper transport protein. The treated rats also performed better on the Barnes maze, a standard test where animals learn to find an escape hole using spatial cues.
The effect was specific to the CA3 region. CA1 and the dentate gyrus showed no change. CA3 handles pattern completion, the process of reconstructing a memory from partial information. Think of it as the difference between recognizing your car in a parking lot versus remembering where you parked. The second task relies heavily on CA3.
The Numbers
In cell culture, L10 showed no toxicity at concentrations up to 500 micromolar. Its EC50 was 914 micromolar, meaning you’d need nearly double that concentration to kill half the cells. Compare that to L11, the quinoline based compound, which had an EC50 of just 68 micromolar. L11 grabbed copper more aggressively but killed cells faster too.
The structural difference between L09 and L10 is one carbon. L09 has a methoxy group; L10 has an ethoxy group. That single carbon makes L10 about 2.6 times more fat soluble, which likely explains its better brain penetration.
BDNF levels, which drop in Alzheimer’s and track with cognitive function, recovered in treated animals. Malondialdehyde, a marker of lipid oxidation, went down. The streptozotocin model rats had accumulated excess intracellular copper; after treatment, levels normalized.
What This Doesn’t Tell Us
The streptozotocin model produces some Alzheimer’s like pathology but it’s a rat, not a human. Translation often fails. Previous copper chelators, including clioquinol and PBT2, looked good in animals and early human trials but never made it to market. Clioquinol had contamination problems and concerns about zinc depletion. PBT2 couldn’t show consistent plaque clearance in larger trials.
The regional specificity is a question mark. Why CA3 and not CA1? The researchers don’t have a clear answer. It could mean the compound only helps certain disease presentations. It could mean five days wasn’t long enough to see broader effects.
Human trials will answer some of this. The team has filed a patent and is looking for pharmaceutical partners.
What We Can Use Now
We can’t prescribe L10. The study does give us a reason to be more systematic about copper assessment in cognitive cases.
Serum copper by itself doesn’t tell you much. Ceruloplasmin carries most of it, so the copper that’s not bound to ceruloplasmin, sometimes called free copper, is the more relevant fraction. An elevated free copper level in a patient with cognitive symptoms should get your attention. The zinc to copper ratio is worth tracking too, since the metals compete for absorption and influence each other’s tissue levels.
The ATP7B finding connects this work to a broader genetic story. Mutations in ATP7B cause Wilson’s disease, where copper accumulates in liver and brain. Full Wilson’s is rare, maybe 1 in 30,000. But Rosanna Squitti’s research group in Italy has spent over a decade showing that ATP7B variants, not the severe mutations that cause Wilson’s but milder polymorphisms, turn up more often in Alzheimer’s patients than in matched controls. In a 2013 study of about 500 subjects, her team found several ATP7B variants associated with AD risk, with odds ratios between 1.5 and 2.3. The variants that showed the strongest association were in the transmembrane domains, the parts of the protein that actually move copper across membranes.
What this suggests: some people are genetically less efficient at clearing copper. They don’t have Wilson’s disease, but they may be more vulnerable to copper accumulation over decades, especially if dietary copper is high or zinc is low. When you see a patient with cognitive decline and elevated free copper, particularly with a family history of unexplained neurological or psychiatric illness, you may be looking at this phenotype.
ATP7B testing is available but not yet standard for cognitive workups. The functional markers, serum copper, ceruloplasmin, and zinc, give you actionable information now. If free copper is high, you have an intervention path: gradual zinc supplementation, antioxidant support, dietary copper reduction, retest in three to six months.
What to Order
| Test | Why |
| Serum copper | Total body copper status |
| Ceruloplasmin | Carrier protein; needed to calculate free copper |
| Plasma zinc | Competes with copper; ratio matters |
Calculate free copper: Total serum copper (mcg/dL) minus (ceruloplasmin mg/dL × 3). Values above 1.6 μmol/L warrant attention.
Available through LabCorp, Quest, Genova, Doctor’s Data. Walsh protocol practitioners and Bredesen-trained clinicians order these routinely.
Why This Research Matters for Naturopathic Practice
Fifty million people have Alzheimer’s disease worldwide. The conventional drug pipeline has produced three acetylcholinesterase inhibitors that offer modest symptomatic help, memantine for moderate to severe cases, and now monoclonal antibodies that can reduce plaques but cause brain swelling and bleeding in a meaningful percentage of patients. Clinical benefit from the antibodies has been marginal despite the plaque reduction.
Naturopathic medicine has always looked at the terrain: oxidative stress, inflammation, toxic burden, nutrient status. Copper fits that picture. We’ve tested it. We’ve thought about it. Now there’s a study showing that removing excess copper from brain tissue reverses disease markers in an animal model. We’ve been asking the right questions.
Alzheimer’s has multiple subtypes with different drivers. Copper dysregulation may matter a lot for some patients and have nothing to do with others. The regional specificity in this study, with CA3 responding and other areas staying flat, hints at that. One protocol won’t fit everyone. For the subset where copper is part of the picture, this research points toward disease modification rather than symptom management.
If the human trials happen and the compound works, it would be the first Alzheimer’s drug to come from the bioinorganic chemistry field. Cerchiaro’s group notes that L10 is cheap to make compared to monoclonal antibodies. Access would be broader.
For now, order the labs and watch the literature.
References
Camargo MLM, Farias AB, Bertazzo GB, et al. Novel Copper Chelators Enhance Spatial Memory and Biochemical Outcomes in Alzheimer’s Disease Model. ACS Chem Neurosci. 2025;16:3267-3281.
Squitti R, Polimanti R, Siotto M, et al. ATP7B Variants as Modulators of Copper Dyshomeostasis in Alzheimer’s Disease. Neuromolecular Med. 2013;15:515-522.
Squitti R, Polimanti R, Bucossi S, et al. Linkage Disequilibrium and Haplotype Analysis of the ATP7B Gene in Alzheimer’s Disease. Rejuvenation Res. 2013;16:3-10.
Agência FAPESP. Researchers develop chemical compound with potential against Alzheimer’s disease. November 12, 2025.