Naturopathic Perspective
Quinn Rivet ND
Anemia of chronic disease (ACD), also known as anemia of inflammation, is typically characterized by a microcytic or normocytic, normochromic anemia with low reticulocytes, along with possible mildly low hematocrit, hemoglobin, and iron levels, but high ferritin.1 ACD affects millions of North Americans with few clinical options, in many cases requiring parenteral iron infusion, which in turn can cause even more inflammation. The clinical phenomenon of ACD results from chronic inflammation brought on by chronic infection, chronic inflammation (eg, Crohn’s disease, autoimmune disease), severe trauma, cancer, kidney failure including those on renal dialysis, and aging.2,3 Considering the etiopathogenic mechanisms of ACD, this article will present its prevalence, pathophysiology, lab findings, and treatment with certain complementary therapies, including the stilbene, resveratrol; the curcuminoid, curcumin; and the organosulphur compound known as thioctic or alpha-lipoic acid.
ACD is the second most prevalent cause of anemia in the general population (iron deficiency is the first2,3), and is the most common cause of anemia in critically ill hospitalized patients.2 ACD increases with age, as was reflected in a 2012 United States National Health and Nutrition Examination Survey (NHANES III), which reported that ACD affected up to 11% of non-institutionalized elderly men and 10.2% of non-institutionalized women.4 ACD is associated with increased disability,4 decreased activities of daily living,4 increased left ventricular hypertrophy,5-7 decreased executive function in women,4 depression,4increased fracture rate in older women,8 increased hospital visits,4 decreased motor performance,3 and increased overall morbidity and mortality.4 Thus, one can see that ACD is not a benign clinical entity, particularly in the elderly or those highly prone to it, eg, patients on hemodialysis, patients with cancer, and those with an active autoimmune or inflammatory disorders.
Table 1. Causes of ACD2
| Associated Diseases | Estimated Prevalence (%) |
| Infections (acute/chronic) | 18-95 |
| Cancer | 30-77 |
| Autoimmune | 8-71 |
| Chronic rejection after solid organ transplant | 8-70 |
| CKD, inflammation, renal dialysis | 25-30 |
(CKD = Chronic kidney disease)
Pathophysiology of ACD
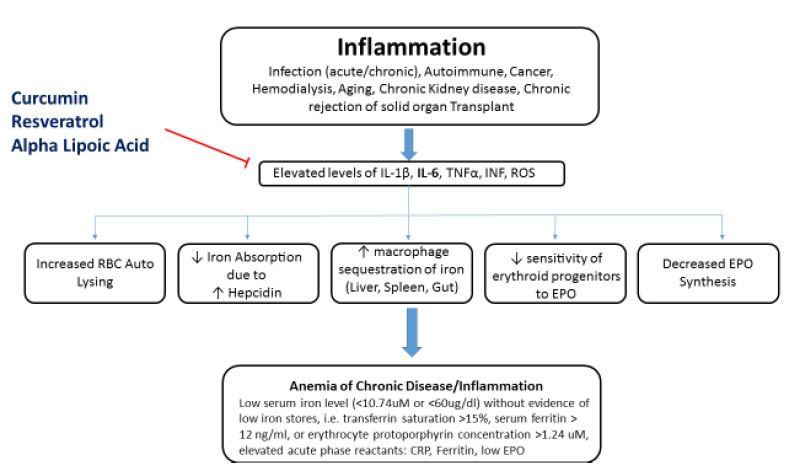
ACD is typically a manifestation of low hematopoietic production of red cells in conjunction with a shortened red blood cell (RBC) lifespan.1-3 Generally, this hypoproliferative state of RBC production is induced by chronic inflammation via several different processes: liver hepcidin production,9 reactive oxygen species (ROS), and inflammatory cytokines – particularly interleukin (IL)-1 and IL-6, tumor necrosis factor (TNF), and interferon (INF).1-3,9-19 These cytokines tend to impair the marrow macrophages’ release of their iron to the erythroid progenitors. Inflammatory cytokines cause progenitor cells to be less sensitive to erythropoietin, resulting in low RBC production.16,18 This understanding becomes of great clinical utility when dealing with refractory recombinant human erythropoietin (rHuEPO) therapy, as controlling inflammatory cytokines can enhance patients’ responsiveness to rHuEPO. These inflammatory cytokines also cause macrophages in the liver, spleen, and gut to sequester iron, thereby decreasing the availability of free iron for hemoglobin production.2,16,17 Inflammatory cytokines and ROS have also been found to decrease the production of erythropoiesis in the kidney itself and to change the response of the erythroid progenitor cells to erythropoietin (EPO).18
Hepcidin
Hepcidin is the body’s main iron-regulatory molecule.9,10 It is synthesized in the liver in response to elevated levels of IL-1β and IL-6.2,9,10 Hepcidin inhibits iron absorption at the brush border of the duodenum by blocking the ferroportin channels.4,5,10 Hepcidin also causes macrophages to sequester iron from hemoglobin and prevents the recycling of hemoglobin from the RBC.4,5,10 Both processes result in an inadequate iron pool for normal hemoglobin levels.
Generally, ACD is brought about by decreased RBC production, increased RBC destruction, and low iron availability. This culminates in a normocytic (possibly microcytic), normochromic (possible hypochromic) anemia within the context of inflammation that occurs in a specific clinical condition or combination of circumstances, eg, chronic kidney disease (CKD) with hemodialysis, or cancer with a chronic upper respiratory infection.2
Lab Findings2
- Normocytic/microcytic and normochromic anemia, although eventually microcytic and hypochromic, similar to that found in iron deficiency anemia
- Slightly low hemoglobin and high reticulocyte count
- High transferrin (not only due to lack of iron, but also due to increased production of transferrin); transferrin saturation is low
- Low serum iron
- Normal or high ferritin (acute-phase reactant), revealing that the body is inflamed
- Elevated C-reactive protein (CRP) (acute-phase reactant)
- Elevated inflammatory cytokines and oxidative stress markers [added by author]
Therapeutics
The best treatment for ACD is to eradicate the underlying disease. When this is not a clinical option, blood transfusions, intravenous iron supplementation, and rHuEPO may help ameliorate the condition. Newer approaches that target TNFα activity and the hepcidin-ferroportin axis have been investigated in a murine model, eg, an anti-TNF antibody.10 The monoclonal TNFα antibody, infliximab, has also been found to reduce anemia in those with inflammatory bowel disease.10,19
Although these newer interventions that target TNFα (eg, infliximab) have potential for wide-ranging clinical utility in ACD/inflammation, they can be very costly. Transfusions and parenteral iron can cause elevated organ iron levels as well as extreme oxidative stress, which in turn can promote possible end-organ damage (eg, heart, kidney, brain, and liver) from the accumulation of iron stores.20-22
Contemporary understanding of the pathophysiology would suggest that clinical applications should target the underlying condition. However, amelioration may not be possible under certain conditions (eg, cancer, hemodialysis, aging), in which case the down-regulation of ROS and the inflammatory cytokines (IL-6, IL-1β, INF, TNF) with appropriate herbals or nutraceuticals is a clinically rational and prudent choice. In the author’s experience, a combination of resveratrol, curcumin, and alpha-lipoic acid can result in very clear and measurable outcomes, as evidenced by changes in the patient’s hematological parameters. Low dosages are typically prescribed, and titrated as required. For example, in hemodialysis patients, dosages should be relatively low, since clearance is low, and only given on dialysis days. A typical prescription includes: 40 mg resveratrol, 500 mg curcumin (95% standardized curcuminoids), and 150 mg alpha-lipoic acid, administered 1 hour before the dialysis procedure. Of course, any foci of inflammation should also be assessed and treated (eg, gums, teeth, gut).
Resveratrol, curcumin, and lipoic acid have been shown in experimental studies to lower ROS and/or inflammatory cytokines IL-1β, IL-6, TNF, and ROS (Figure 1).23-45 Decreasing IL-6 has been shown to reduce hepcidin production.2,26 The lowering of hepcidin by curcumin was reported by Laine et al in a 2017 placebo-controlled, randomized, double-blind, crossover study reported in the Journal of Fundamentals of Clinical Pharmacology.26 The authors concluded that the findings support the use of curcumin for the treatment of anemia of inflammation. Although large human trials investigating the clinical utility of such an application for ACD have not been completed, the author’s experience in treating patients, especially those on hemodialysis, has consistently produced positive clinical outcomes. An interesting adjunctive observation in using this therapy has been a decrease in the required dosage of rHuEPO. A lower rHuEPO dosage is advantageous in that this reduces certain biochemical burdens in the patient as well as the economic burden of such an expensive therapy. Importantly, both lipoic acid and curcumin in high doses (eg, >3 g/day) can chelate iron in the gut, resulting in less iron absorption; in fact, if given for up to 12 weeks with food, curcumin may induce iron deficiency.44 Thus, it is recommended that they be used in small dosages and away from meals. Also, long-term use of lipoic acid in hemodialysis patients has in some cases produced elevated parathyroid hormone, and should therefore not be used continuously, or should be discontinued if parathyroid levels rise. Testing for inflammatory markers will help guide the temporal path of therapy.
Figure 1. Effects of Curcumin, Resveratrol, & Lipoic Acid on ACD
Expected Outcomes
Although other possible alternatives and/or complementary applications exist (eg, ashwagandha, ginger, ginkgo, fermented papaya, milk thistle, N-acetylcysteine, or IV vitamin C for hemodialysis patients46,47), the author has consistently used the titled combination in clinical practice for ACD. Typically, hematological changes can be seen as soon as 14-21 days into therapy. In hemodialysis patients for example, who tend to have extracorporeal mediated immune activation, it may take longer to see changes, especially if parenteral iron is continued (although this will increase hemoglobin, it also exacerbates inflammation, and thus an acute rise in ferritin and CRP may follow).
Resveratrol has lipophilic properties.48 However, there are conflicting reports on whether taking resveratrol with fat influences its absorption. Although one study suggested that taking resveratrol with a fatty meal can inhibit its absorption (thus possibly indicating a need for dosages as high as 2000 mg),49 other research suggests negligible impact of dietary fat on its absorption.50 Further research is needed for clarification.
Combining the 3 substances (resveratrol, curcumin, lipoic acid) may have a synergistic effect by enhancing their absorption, thus allowing lower doses of each. Hematological follow-up studies should reveal drops in ferritin (since it is an acute-phase reactant), CRP, IL-6, oxidative stress markers, and increases in hemoglobin and hematocrit. Improvements in hemoglobin and hematocrit will support a better quality of life as a result of increased cognition, endurance, mood, and overall activities of daily living.
Note: Hemodialysis patients are managed with a specific hemoglobin target level (11-12 g/dL, or 110-120 g/L) in order to decrease the risk of fistula thrombosis. Higher is not necessarily better; studies favor lower hemoglobin in this patient population.51
Conclusion
Anemia of chronic disease/inflammation is a prevalent clinical entity throughout the North American population, resulting in significant morbidity and mortality. Medical treatment is limited, is expensive, and can lead to unwanted side effects, eg, iron accumulation from intravenous iron. Contemporary treatment relies on parenteral iron infusions, blood transfusion, and rHuEPO, which may alleviate the anemia; however, this approach increases the possibility of instigating further biological perturbations as well as being a heavy economic burden [note that in Canada we do not pay for health care, and thus I just mentioned a heavy economic burden, referring to government and insurance economic burden]. The therapeutic integration and application of alpha-lipoic acid, curcumin, and resveratrol have been shown in certain clinical settings to ameliorate inflammation, and thus anemia associated with chronic inflammation. Although human clinical trials are required to fully assess this application, the use of these substances may open new vistas for their clinical utility in anemia of chronic disease, particularly in the geriatric and hemodialysis population.
References:
- ArupConsult. Anemia of Chronic Disease – Anemia of Inflammation. Last updated June 2019. Available at: https://arupconsult.com/content/anemia-chronic-disease-anemia-inflammation. Accessed October 20, 2019.
- Madu AJ, Ughasoro MD. Anaemia of Chronic Disease: An In-Depth Review. Med Princ Pract. 2017;26(1):1-9.
- Fraenkel PG. Anemia of Inflammation: A Review. Med Clin North Am. 2017;101(2):285-296.
- Maccio A, Maddedu C, et al. Management of anemia of inflammation in the elderly. Anemia. 2012;2012:56325.
- Metivier F, Marchais SJ, Guerin AP, et al. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15(Suppl 3):14-18.
- Chang JM, Chen SC, Huang JC, et al. Anemia and left ventricular hypertrophy with renal function decline and cardiovascular events in chronic kidney disease. Am J Med Sci. 2014;347(3):183-189.
- Song S, Li G. The Cardiomyopathy of Iron Deficiency Anaemia. Eur Med J. 2018;October 11:92-98.
- Chen Z, Thomson CA, Aickin M, et al. The relationship between incidence of fractures and anemia in older multiethnic women. J Am Geriatr Soc. 2010;58(12):2337-2344.
- Coyne DW. Hepcidin: clinical utility as a diagnostic tool and therapeutic target. Kidney Int. 2011;80(3):240-244.
- Langer AL, Ginzburg YZ. Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment of anemia of chronic inflammation. Hemodial Int. 2017;21 Suppl 1:S37-S46.
- Capocasale RJ, Makropoulos DA, Achuthanandam R, et al. Myelodysplasia and anemia of chronic disease in human tumor necrosis factor-alpha transgenic mice. Cytometry A. 2008;73(2):148-159.
- De Lima GA, Mazzali M, Gentil AF, et al. Anemia in chronic renal disease: evaluation of inflammatory activity on erythropoiesis and iron metabolism in patients not submitted to dialysis treatment. Clin Lab. 2012;58(7-8):695-704.
- Raj DS. Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 2009;38(5):382-388.
- Galushko EA. The clinical significance of hepcidin detection in patients with anemia and rheumatoid arthritis. Klin Med (Mosk). 2014;92(6):21-27. [Article in Russian]
- Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271-1276.
- Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest. 2004;113(9):1251-1253.
- Agus Somia IK, Merati TP, Bakta IM, et al. High levels of serum IL-6 and serum hepcidin and low CD4 cell count were risk factors of anemia of chronic disease in HIV patients on the combination of antiretroviral therapy. HIV AIDS (Auckl). 2019;11:133-139.
- Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28(4):671-681.
- Bergamaschi G, Di Sabatino A, Albertini R, et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010;95(2):199-205.
- Qunibi WY. The efficacy and safety of current intravenous iron preparations for the management of iron-deficiency anemia: a review. Arzneimittelforschung. 2010;60(6a):399-412.
- Rostoker G. When should iron supplementation in dialysis patients be avoided, minimized or withdrawn? Semin Dial. 2019;32(1):22-29.
- Restoker G, Vaziri ND. Risk of iron overload with chronic indiscriminate use of intravenous iron products in ESRD and IBD populations. Heliyon. 2019;5(7):e02045.
- Derosa G, Maffiolo P, Simental-Mendia LE, et al. Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;111:394-404.
- Sahebkar A, Cicero AFG, Simental-Mendia, et al. Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;107:234-242.
- Ghandadi M, Sahebkar A. Curcumin: An Effective Inhibitor of Interleukin-6. Curr Pharm Des. 2017;23(6):921-931.
- Lainé F, Laviolle B, Bardou-Jacquet E, et al. Curcuma decreases serum hepcidin levels in healthy volunteers: a placebo-controlled, randomized, double-blind, cross-over study. Fundam Clin Pharmacol. 2017;31(5):567-573.
- Buhrmann C, Mobasheri A, Busch F, et al. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. 2011;286(32):28556-28566.
- Chin KY. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Devel Ther. 2016;10:3029-3042.
- Chen M, Hu DN, Pan Z, et al. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 2010;90(3):437-443.
- Alisi IO, Uzairu A, Abechi SE, Idris SO. Evaluation of the antioxidant properties of curcumin derivatives by genetic function algorithm. J Adv Res. 2018;12:47-54.
- Mendes PR, Félix Ddos S, Silva PC, et al. Effect of alpha-lipoic acid on the blood cell count and iron kinetics in hypertensive patients. Nutr Hosp. 2014;31(2):883-889.
- Rameriz-Garza SL, Laveriano-Santos EP, Marhuenda-Muñoz M, et al. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients. 2018;10(12). pii: E1892. doi: 10.3390/nu10121892.
- Limagne E, Lançon A, Delmas D, et al. Resveratrol Interferes with IL1-β-Induced Pro-Inflammatory Paracrine Interaction between Primary Chondrocytes and Macrophages. Nutrients. 2016;8(5). pii: E280. doi: 10.3390/nu8050280.
- Yang T, Zhang J, Zhou J, et al. Resveratrol inhibits Interleukin-6 induced invasion of human gastric cancer cells. Biomed Pharmacother. 2018;99:766-773.
- Wang H, Zhang H, Tang L, et al. Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology. 2013;303:139-146.
- Kloesch B, Broell J, Dietersdorfer E, Kiener H. The polyphenols curcumin and resveratrol effectively block IL-1β and PMA-induced IL-6, IL-8 and VEGF-A expression in human rheumatoid synovial fibroblasts. Ann Rheum Dis. 2012;71(Suppl 1):A90-A91.
- BaGen H, Liu X, Jan J. The anti-inflammation effects of resveratrol for patients after oral implantology. Biomed Res. 2018;29(9):1841-1844.
- Lee MH, Kundu JK, Keum YS, et al. Resveratrol Inhibits IL-6 Induced Transcriptional Activity of AR and STAT3 in Human Prostate cancer LNCaP-FGC Cells. Biomol Ther (Seoul). 2014;22(5):426-430.
- Busch F, Mobasheri A, Shayan P, et al. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J Biol Chem. 2012;287(45):38050-38063.
- Dinicola S, Proietti S, Cucina A, et al. Alpha-Lipoic Acid Downregulates IL-1β and IL-6 by DNA Hypermethylation in SK-N-BE Neuroblastoma Cells. Antioxidants (Basel). 2017;6(4). pii: E74. doi: 10.3390/antiox6040074.
- Li G, Fu J, Zhao Y, et al. Alpha-lipoic acid exerts anti-inflammatory effects on lipopolysaccharide-stimulated rat mesangial cells via inhibition of nuclear factor kappa B (NF-κB) signaling pathway. Inflammation. 2015;38(2):510-519.
- El-Nakib GA, Mostafa TM, Abbas TM, et al. Role of alpha-lipoic acid in the management of anemia in patients with chronic renal failure undergoing hemodialysis. Int J Nephrol Renovasc Dis. 2013;6:161-168.
- Showkat A, Bastnagel WR, Hudson JQ. Effect of α -Lipoic Acid on Oxidative Stress in End-Stage Renal Disease Patients Receiving Intravenous Iron. ISRN Nephrol. 2014;2014:634515.
- Smith TJ, Ashar BH. Iron Deficiency Anemia Due to High-dose Turmeric. Cureus. 2019;11(1):e3858.
- Tabrizi R, Tamtaji OR, Lankarani KB, et al. The effects of resveratrol supplementation on biomarkers of inflammation and oxidative stress among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 2018;9(12):6116-1628.
- Attallah N, Osman-Malik Y, Frinak S, Besarab A. Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia. Am J Kidney Dis. 2006;47(4):644-654.
- Shahrbanoo K, Taziki O. Effect of intravenous ascorbic acid in hemodialysis patients with anemia and hyperferritinemia. Saudi J Kidney Dis Transpl. 2008;19(6):933-936.
- Gambini J, Inglés M, Olaso G, et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid Med Cell Longev. 2015;2015:837042.
- la Porte C, Voduc N, Zhang G, et al. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49(7):449-454.
- Vitaglione P, Sforza S, Galaverna G, et al. Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res. 2005;49(5):495-504.
- Ye Y, Liu H, Chen Y, et al. Hemoglobin targets for the anemia in patients with dialysis-dependent chronic kidney disease: a meta-analysis of randomized, controlled trials. Ren Fail. 2018;40(1):671-679.
 Quinn Rivet, ND, graduated from CCNM in 1994. From 2001 to 2012 he was an instructor, clinical supervisor, and the chair of nutrition (2004-2010) at BINM. Dr Rivet was in clinical practice for 23 years. He has relocated to China, were he is the principle director of a high school program. This serves to subsidize his research, writing, and exploration of TCM, as well as the investigation of possible environmental triggers related to the high prevalence of IgA nephropathy in China. Dr Rivet can be contacted through his web site: drquinn-nd.com.
Quinn Rivet, ND, graduated from CCNM in 1994. From 2001 to 2012 he was an instructor, clinical supervisor, and the chair of nutrition (2004-2010) at BINM. Dr Rivet was in clinical practice for 23 years. He has relocated to China, were he is the principle director of a high school program. This serves to subsidize his research, writing, and exploration of TCM, as well as the investigation of possible environmental triggers related to the high prevalence of IgA nephropathy in China. Dr Rivet can be contacted through his web site: drquinn-nd.com.