Post-Pandemic Clinical Medicine: Strategies for Treating Long-COVID and Vaccine-Related Injuries
Paul S. Anderson, NMD
A comprehensive review of definitions, pathogenesis, diagnostics, and evidence-based therapeutic approaches for managing Long-COVID and post-vaccination syndromes in clinical practice.
Abstract
Long-COVID (LC) and Post-Vaccination Syndromes (PV) have emerged as overlapping and increasingly common clinical conditions. This review synthesizes current evidence on definitions, incidence, pathogenesis, and therapeutic strategies, highlighting multi-omic findings, immune dysregulation, and “spikeopathy” as key drivers of pathology. Clinical assessment frameworks, including the Klok Post-COVID Functional Status scale, are presented alongside laboratory and dysfunction-based approaches to diagnosis. Evidence-based therapeutic strategies—ranging from spike-protein degradation and immune modulation to mitochondrial and coagulation support—are outlined for each severity grade. This review emphasizes patient-centered, individualized management while acknowledging the complexity and chronicity of LC and PV cases.
Introduction
The clinical management of patients with Long-COVID (LC) and Vaccine Injuries / Post-Vaccination effects (PV) has emerged as a significant and growing need in modern medical practice.
While published data vary regarding incidence, mechanisms, pathogenesis, and therapeutic strategies, there is no doubt that these clinical entities exist and represent a substantial portion of daily patient interactions. Assessment and clinical management strategies are greatly needed for both LC and PV. Those are the focus of this review of the published data, guidelines, and clinical practice.
A legitimate question I receive is, “I didn’t think you practiced anymore—how are you qualified to address any of this?” First, I do still maintain a full patient practice, though I do not publicly advertise it. My caseload is roughly split equally into oncology patients and complex chronic illness patients. Second, during the early COVID-19 pandemic —when severe illness and hospitalization were widespread – I was actively involved in patient care. This included home-based acute care and follow-up for COVID patients, as well as consulting with ICU physicians regarding implementation of the hospital-based intravenous vitamin C protocol I had published. 1,2
Because of this direct and indirect patient care with COVID patients, I became part of a number of physician groups who shared response data for therapies used during COVID infection, as well as LC and PV therapeutics. Collectively, these collaborations generated comparative data on more than 2,000 patients, providing valuable insights into clinical response patterns and effective interventions.
Presentation:
Definitions:
Long-COVID (LC):
“Long COVID has been reported in at least 10% of patients recovering from SARS-CoV-2 infections and possibly up to 50% to 70% of hospitalized cases. This condition is seen in all levels of disease severity, from asymptomatic to critically ill, but the likelihood of this condition is highly correlated with the severity of symptoms. More than 200 symptoms have been associated with this illness, with the most commonly reported clinical symptoms being malaise, dyspnea, fatigue, cognitive impairment (“brain fog”), autonomic dysfunction, headache, persistent loss of smell or taste, cough, depression, low-grade fevers, palpitations, dizziness, muscle pain, and joint pains.”3
The National Academy of Sciences also has an extensive publication, “A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences,” which is well-referenced and far-reaching. This document, however, is more targeted toward defining future research and public health policy.4
Post-Vaccination Syndromes (PV)
“Disease definition for post-COVID-19 vaccination syndrome (PCVS) has been challenging, owing to different clinical presentations. The terminology ‘acute COVID-19 vaccination syndrome’ (ACVS) is adopted to refer to adverse events that occur soon (immediately after up to days) after vaccination. The terminology ‘post-acute covid-19 vaccination syndrome’ (PACVS) refers to a different clinical presentation than PCVS, and refers to lingering symptoms that typically last longer than one month and may last for years.
Clinically, PACVS presents similarly to another disease cluster, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Other overlaps with existing syndromes include postural orthostatic syndrome (POTS), mast cell activation syndrome (MCAS), and small fibre neuropathy (SFN). PACVS is characterized by dysautonomia, post-exertional malaise, fatigue, and often accompanying neuropathic pain and cognitive disturbances, such as brain fog.”5
A systematic review and meta‐analysis of symptoms 3 Years post‐SARS‐CoV‐2 Infection published in 2025 found the following for LC: “Pooled analysis showed that the proportion of individuals experiencing at least one persistent symptom 3 years post‐COVID‐19 is 20% (95% confidence interval [CI]: 8–43). The prevalence of persistent symptoms was dyspnea (12%; 95% CI: 10–15), fatigue (11%; 95% CI: 6–20), insomnia (11%; 95% CI: 2–37), loss of smell (7%; 95% CI: 5–8), loss of taste (7%; 95% CI: 3–16), and anxiety (6%; 95% CI: 1–32). Prevalence of other findings includes impaired diffusion capacity (42%; 95% CI: 34–50) and impaired forced expiratory volume in 1 s (10%; 95% CI: 8–12). Our findings confirm the persistence of unresolved symptoms 3 years post‐COVID‐19 infection.”6
Comparing this presentation to published PV data shows a great deal of crossover. PV presentations include “symptoms such as dysautonomia, post-exertional malaise, fatigue, neuropathic pain, and cognitive disturbances, overlapping with syndromes like myalgic encephalomyelitis/chronic fatigue syndrome, postural orthostatic syndrome, and small fiber neuropathy.”5 Neurological effects are wide reaching and summarized as “spike proteins are able to cross the blood brain barrier (BBB) and can trigger acute and chronic neurological complaints. Such SP-associated pathologies (spikeopathies) are further neurological proteinopathies with thrombogenic, neurotoxic, neuroinflammatory, and neurodegenerative potential for the human nervous system, particularly the central nervous system.”7
Considerations in Pathogenesis and Severity:
Both LC and PV share many clinical features as noted above. In addition to this, they can also coexist and become co-pathogenic triggers. Owing to this clinical “collage effect” where one or both entities may be present, a brief summary of factors aggravating the disorders will follow to include possibly individual LC or PV, or a combination of both. A partial list of factors increasing the incidence of LC, PV, or both can include:
- History of hospitalization for COVID6,11
- Cigarette smoking11
- Substance abuse11
- Comorbid infections12-16,24-29
- Endocrinopathies17,18
- More severe presentation of illness or symptoms11
- POTS/ Dysautonomia, ME-CFS19,32
- Autoimmunity19-21
- Mold – Mycotoxin22,23
Multi-Omics data findings:
Multi-omic (or multiomic) data assessment is a holistic manner of assessing complex systems and information. It is an integrated analysis of multiple biological datasets, or “omes,” to gain a more complete understanding of complex biological systems and disease processes. It combines data from omics technologies like genomics, transcriptomics, proteomics, epigenomics, and metabolomics
“The synthesis of omics data, including genomics, transcriptomics, proteomics, metabolomics, and metagenomics, revealed common findings associated with fatigue, cardiovascular, pulmonary, neurological, and gastrointestinal phenotypes. Key findings included mitochondrial dysfunction, dysregulated microRNAs associated with pulmonary dysfunction, tissue impairment, blood–brain barrier disruption, coagulopathy, vascular dysfunction, microbiome disturbances, microbial-derived metabolite production, and persistent inflammation.”30 And: “Overall, our findings provide genomic evidence consistent with previous epidemiological and clinical reports of long COVID, indicating that long COVID, similarly to other postviral conditions, is a heterogeneous disease entity where likely both individual genetic variants and the environmental risk factors contribute to disease risk.”31
Incidence and Frequency?
The incidence of people affected by either LC or PV is published, but also constantly changing. A review of almost any cited publication, and specifically 5,6,8,9,10, will underscore the fact that the state of the data regarding incidence is fluid, at best.
As the focus of this review is to elucidate diagnostic and therapeutic directions, the fluid nature of incidence data will be left as is. If one sees one or one hundred patients with LC or PV, the statistical incidence of those issues overall is relatively meaningless.
Whether more or less common, both LC and PV are common clinical entities, and their effects should be addressed in patients as they come to healthcare providers.
“Spikeopathy” as a modern pathology:
“Spike protein pathogenicity, termed ‘spikeopathy’, whether from the SARS-CoV-2 virus or produced by vaccine gene codes, akin to a ‘synthetic virus’, is increasingly understood in terms of molecular biology and pathophysiology. Pharmacokinetic transfection through body tissues distant from the injection site by lipid-nanoparticles or viral-vector carriers means that ‘spikeopathy’ can affect many organs. The inflammatory properties of the nanoparticles used to ferry mRNA, N1-methylpseudouridine employed to prolong synthetic mRNA function; the widespread biodistribution of the mRNA and DNA codes and translated spike proteins, and autoimmunity via human production of foreign proteins, contribute to harmful effects.”35
“Spikeopathy” from vaccine or viral infection can affect almost any body system:
- Triggers cytokine shifts that imbalance immune function and activate microglia36
- Triggers clotting abnormalities, including microclotting, via PAF and other inflammatory cytokines36-38,54,56-62
- Creates brain inflammation equal to a traumatic brain injury36,39,41,42
- Triggers Mast-cell and Mast-cell mediators33,38-40
- Triggers underlying changes, making both LC and PV syndromes more likely40,43,55
- Triggers underlying changes, making cancer more likely44-53
Are their data showing that symptoms of LC or PV can be triggered by vaccine versus infection, and how do they know?
Data exist that show that the spike protein has damaging potential to body tissues, regardless of the source being infection or vaccine: “spike protein by itself (without being part of the coronavirus) can damage endothelial cells and disrupt the blood-brain barrier”.33
In a paper specifically looking at patients with Lupus (SLE), it was found that the N protein can create inflammatory changes and damage in the absence of active COVID infection.64 The authors report that “the SARS-CoV-2 N protein can independently trigger sustained type I IFN production via direct activation of MAVS* and its spontaneous oligomerization. This long-lasting type I IFN generation may create a chronic inflammatory milieu, favoring autoimmunity with SLE-like symptoms in susceptible individuals.”
[*MAVS: The mitochondrial adaptor protein “MAVS” is a key regulator of IFN-I]
Some data exist showing the potential for spike protein from vaccination to trigger systemic pathology: “In patients ≥76 years, COVID-19 vaccines were protective for the occurrence of myocardial injury, while in patients ≤60 years, myocardial injury was associated with previous COVID-19 vaccination.”34 Other publications have asserted that there are vaccine-induced conditions, including:
- Post-COVID-19 vaccine Syndrome (PCVS) is hallmarked by increased inflammatory mediators.67
- The use of mRNA vaccines in people with immune compromise (regardless of cause) showed no additional benefit for prevention or protection from illness.69
- An increase in IgG4 to the spike protein following the second or more mRNA vaccinations triggers immune tolerance to the virus with suppressed natural immunity and increased “breakthrough” infections.68
- Such tolerance and immune alterations are also permissive to the development of autoimmunity, myocarditis, and cancer development.68
Autopsy data are limited, but one publication reviewing multiple studies showed patterns of disease states in people dying within two weeks of vaccination:70 The Hulscher et al. systematic review examined 325 autopsy cases from 44 published studies, finding that 73.9% of deaths were adjudicated by independent physicians as being directly caused by or significantly linked to COVID-19 vaccination. The leading causes of death included:
– Sudden cardiac death (35%)
– Pulmonary embolism (12.5%)
– Myocardial infarction (12%)
– Vaccine-induced immune thrombotic thrombocytopenia (VITT, 7.9%)
– Myocarditis (7.1%)
– Multisystem inflammatory syndrome (4.6%)
– Cerebral hemorrhage (3.8%)
Is it possible to detect vaccine spike presence versus infection-produced spike?
The short answer is yes. It is possible to use laboratory technology to determine if the spike protein being measured is from a natural COVID infection versus vaccination.
Various methodologies, such as receptor binding domain-specific IgG3 to the S, and IgG to nucleocapsid [N].63
In another study of 50 individuals with Post COVID Vaccine Syndrome (PCVS) symptoms lasting over 30 days post-vaccination and 26 asymptomatic controls, the use of anti-N, T-detect assays, machine learning-based immune profiling to compare cytokine signatures with PASC, flow cytometry to detect S1 in CD16+ monocytes, and LC-MS to confirm S1 across vaccine types and control groups correlated S1 persistence with PCVS symptom duration and inflammation. Vaccine-triggered S1 spike protein in CD16+ monocytes was found up to 245 days after vaccination (Prior infection was excluded via clinical history, anti-nucleocapsid antibody tests, and T-detect assays). Preliminary findings suggest S1 from vaccine persistence in CD16+ monocytes and an associated inflammatory profile may contribute to PCVS.67
If this is true, how does this relate to the commonly available laboratory tests in North America?
While there are multiple iterations of tests for COVID antibodies available, the commonly used tests for LC and PV are:
- SARS-CoV-2 Semi-Quantitative Total Antibody, Spike [S-Protein, Semi-quant]
- SARS-CoV-2 Antibodies, Nucleocapsid [N-Protein, Qualitative]
The S-Protein test reflects immunoglobulin levels to the spike, which could be due to infection, vaccination, or both. It does NOT give an actual count of spike protein, but rather a level of immunoglobulin response.
The N-Protein test measures a qualitative (“yes – no”) level of presence or absence of the nucleocapsid. This is ONLY positive due to COVID infection, and not vaccination.
The importance of using an N-Protein test is to assess the presence or absence of COVID infection due to the virus. This has been the basis of some control studies looking at vaccine effects in non-COVID-infected persons.64,67
The S-Protein test can create some confusion in that it does not give a quantification of actual spike protein but rather the immunoglobulin response to that spike, and also that it may not be clear if an elevation in this value is indicative of vaccine-derived spike, infection-derived spike, or both.
The “normal” for this test is less than 0.8 Units / mL (U / mL). Many clinicians have anecdotally pointed out that in healthy patients who have a history of COVID infection, their levels of S-Protein on this test may be 100 – 800 U / mL, but numbers wane as time progresses (unless the person has a re-infection). This is in contrast to similar clinician reports that those with LC or PV illnesses have values of 800 – 1000 and up to > 25,000 U / mL, which often do not wane over time if the LC or PV symptom picture persists.
Are there any data that support these anecdotal observations? Yes. The post-infectious drop in spike antibodies is significant at 90 days post-infection, and continues to drop after that, provided no re-infection occurs.66 Post-vaccine levels of the spike antibodies in those with no history of COVID infection were at an average of 3600 U / mL at two years post-vaccine and at an average of 7440 U / mL after boosters beyond vaccine-2.
Is it possible to obtain an actual assay of spike proteins in a sample that is not an immunoglobulin level, as noted in the common North American tests above? It is possible in two settings. One is in a university lab center performing COVID research. The other is by using commercial laboratories currently outside the US. Some labs can detect S-Protein, Modified mRNA, and Plasmid-DNA.71
Finally, some clinicians are using cytokine-based immune pan
els which do not measure any COVID protein but rather inflammatory chemistry in specific patients with LC or PV. These tests are used to follow the immunochemical trajectory of the patient case.
Summary:
In summary, it is wise to remember that the needs of the patient come before assigning blame to a pathogenic trigger. And while LC and PV can be independent entities, often they coexist and create a greater pathogenic effect in combination.
Assessment and Initial Interventions:
For either LC or PV assessment, there are two broad methods of assessment those being direct trigger assessment (Spike-protein, cytokine, etc.) and whole-case comorbidity assessment. Both will be outlined below.
Spike Protein / Cytokine Assessment:
SARS-CoV-2 Semi-Quantitative Total Antibody, Spike [S-Protein, Semi-quant]
SARS-CoV-2 Antibodies, Nucleocapsid [N-Protein, Qualitative]
Recall from the discussion above that the “N” protein is a marker of COVID infection and may be helpful in differentiating infection-triggered versus vaccine-triggered spike levels.
In the discussion above, the level of “S” protein differs between COVID infection and post-vaccine reaction. COVID infection triggered “S” rises to 500 – 800 U / mL and begins to fall after 90 days. Vaccine-induced “S” can be 800 – 1000 to over 25,000 U / mL and has been noted in the data at 245 days.67
Some labs in Europe can detect S-Protein, Modified mRNA, and Plasmid-DNA. 71
Some labs test individual cytokines using data regarding cytokine-based pathophysiology of Long COVID symptoms that may include IL-1 series, IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-g, and IL-17A.72
Other publications recommend the following markers: IL-2, 4, 6, 8, 10, 13 GM-CSF, IFN-g, VEGF, (s)CD40l, CCL 3, 4, 5.80
Dysfunction-based assessment and treatment:
In order to assess the level of dysfunction experienced by the patient with LC or PV, a grading system should be employed. This allows the clinician to stratify their diagnostic and therapeutic approach while prioritizing appropriate laboratory and other interventions.
Does it matter if one is assessing and treating PV or LC? In many senses, no. As the conditions are often overlapping, and both have influences from comorbid conditions beyond COVID or Spike protein, the approach to assessment and therapeutics can begin in the same place and be personalized as required.
The simplest and most clinically useful scale for either LC or PV I have found was published by Klok et. al. In the discussion of assessments below, I will use this scale to stratify the clinical workup details.
Multiple papers outline the comorbidities of LC and PV syndromes. These include endocrine deregulation, co-infections, coagulopathies, de novo and aggravated autoimmunity, increased sensitivity to environmental toxicants, potential for oncogenic processes, and many others.13,15,16,19,23,30,31,75-79 It is with this plethora of triggering and aggravating factors that the following stepwise assessment protocol was created. Please note that these are only starting places and may be altered as clinically indicated.
Graphic Below from: “Klok FA, Boon GJAM, Barco S, et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J 2020; 56: 2001494”
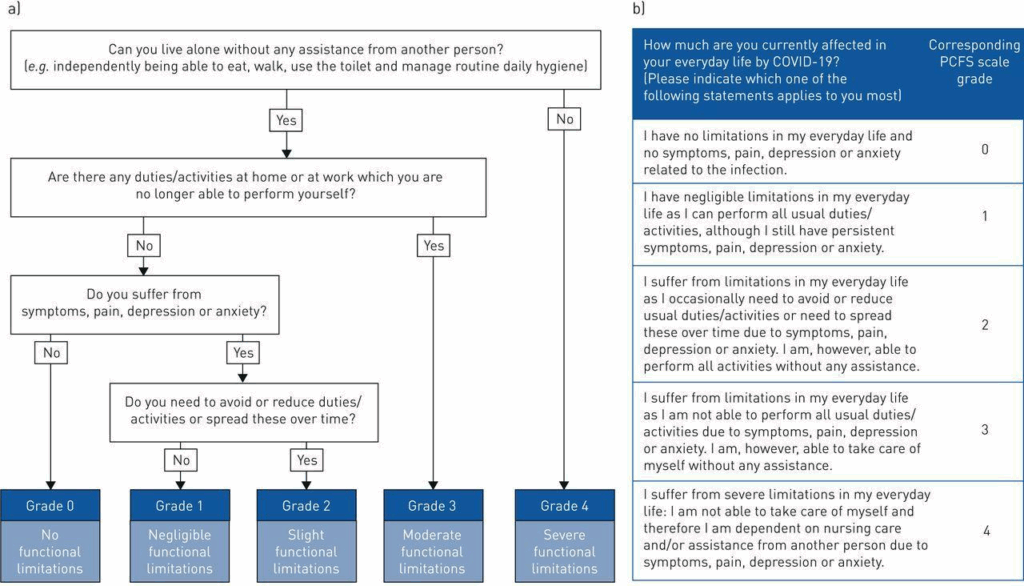
Initial History: LC or PV
- Current Sn/Sx and effect on ADL (see the “Klok” scale above)
- Past History (active or inactive):
- CFS, FMS, or other unexplained fatigue states
- Chronic Infections, Inflammatory (PANS, CIRS…)
- Autoimmunity or other Chronic Illness
- Chronic GI disease/syndromes
- Cancer
- Pain syndromes, migraine, etc.
- Treatment for toxicity (mold, chemical, metal)
- Use of immunosuppressive therapies
- Clotting Disorders
- Neuropsychiatric History
- Any other relevant pre-existing condition
- COVID vaccine, type and number
Assessment Targets: LC or PV
- Past History
- Mitochondrial Damage
- BBB – Neuroinflammation
- Clotting
- Inflammation
- Endocrine Dysregulation
- Infectious Agents & Resistance Factors
- GI Dysregulation
- Toxins
- Genomics
- Hallmarks, signs, or indications of occult or a known progressing cancer.44-53,68
Clinical Dysfunction-Based Assessment and Treatment Ideas
Klok Asymptomatic Grade – 0
- Monitor basic labs: CBC, Ferritin, CMP-14, CRP, D-dimer, AlkPhos, LDH, CPK
- As a baseline: SARS-CoV-2 Semi-Quantitative Total Antibody, Spike [S-Protein, Semi-quant]
- If assessing presence of antibodies from infection: SARS-CoV-2 Antibodies, Nucleocapsid [N-Protein, Qualitative]
- Monitor any pre-existing abnormal labs (i.e., HbA1C, Thyroid…)
- Rx: Exercise, Low Inflammation Diet, Centering/Meditation, Hydration
- Implement or keep them on the basic nutritional interventions:
- Vit. A, D/K2, E; Vit. C, B-Complex, Multimineral …
- Treat acute issues PRN
- Taper to baseline supplements in 2-3 months
- Have them contact you if the situation changes.
Klok Mild Grade 1 – 2
- The above-listed “Klok Grade-0” labs and assessments plus:
- Re-test any pre-existing conditions
- Increase coagulopathy assessment to include D-dimer (quantitative), Fibrinogen activity, Platelet Count, PT-INR
- Expanded Thrombosis, Venous Risk Profiles may be necessary in more advanced cases can include:
- Anticardiolipin antibodies, IgG and IgM
- Antithrombin activity
- β2-glycoprotein 1 antibodies, IgA, IgG, and IgM
- D-dimer
- dRVVT screen
- Factor II
- (prothrombin) mutation analysis
- Factor V Leiden mutation analysis
- Protein C, functional
- Protein S antigen, free
- Expanded Thrombosis, Venous Risk Profiles may be necessary in more advanced cases can include:
- Calculate Neutrophil/Lymphocyte ratio and follow as a serial marker (higher NLR = worse inflammation)73
- Add: RBC Mineral Profile or at least RBC Zn, Cu, Mg
- Add: GGT; ANA with Reflex
- Endo: rT3/Ft3/4, Thyroid Ab’s, TSH / AM Cortisol / Consider Estrogen / Progesterone / Testosterone
- ID: ASO, Chlamydia and Mycoplasma pneumoniae, Candida, EBV, Total Ig-G/M/A/E (others as clinically indicated – especially if prior positive.)
- Unchallenged Urine Toxic Metals (UTM) (norm values to NHANES)74, Consider Mycotoxin and Chemical toxin screening
- Empiric therapies:
- Treat any abnormalities found in initial labs.
- Neurosteroid (low first-pass progesterone or pregnenolone). Use of micronized preparations in oil, sublingual drops, tablets, or troches. Doses of 100 to 200 mg nightly are common.
- Immune-balancing:
- Balance 25 (OH) and 1,25 (OH) Vitamin D3 levels
- Immune-focused mushroom therapies, if indicated
- Low Dose Naltrexone
- Ivermectin 0.2 to 0.4 mg/kg [see notes below]
- Monitor q-2-4 weeks in follow-up
Klok Moderate Grade – 3
- All the above-listed labs and assessments in “Klok Grade 0, 1, 2” plus:
- Aggressively treat Endo / ID / Toxic and other cause areas.
- Add on:
- Other infectious screening: Lyme and Coinfections, Other Viri, GI Testing for all infections
- OAT
- Post challenge UTM (you already have a “pre” from above), Mycotoxin and Chemical Toxin screening
- Add on, if not already included:
- Neurosteroid therapy and neurorepair / immunomodulatory immunotherapies, as noted above
- LDN Rx.
- Phase-2 Biofilm Rx.82
- HBOT, if they can gain access, either HBOT level will work:
- 1.3 – 1.5 ATA 60-90 min with 3-4L O2
- or 2.0 – 2.75 ATA with O2 by mask 15L (may need air-break mid therapy) 2-5X a week initially.
- Any COVID or post-COVID patient would benefit from HBOT*
- Other Rx. as indicated
- Consider mitochondrial therapies as needed, such as NAD, Methylene Blue, Red / Near IR Light Therapy, etc.
- Monitor closely.
Klok Severe Grade – 4
- In my (and others’) experience, you cannot “phase in” these people’s work-up.
- A very critical look at any past illness or syndrome, and follow-up is required.
- All areas listed for Endo, Tox, ID, etc. must be assessed and aggressively treated.
- The longer they have had LC or PV Sn/Sx and the more past/comorbidity, the longer to treat. But they will get better if you are aggressive.
Additional Therapeutics:
The above dysfunction-based strategies are the best place to start as far as assessment and basic treatment are concerned.
For both LC and PV, the following areas are critical to consider
- Identify, Treat, and Support Comorbidities
- Support Clotting / Coagulation Issues
- Block Spike-protein from entering cells
- Degrade Spike-protein
- Modulate Immune Function
- Support Antioxidant and Nutrient Status
- Support Mitochondrial Function
Below are some therapeutic ideas that have repeatedly shown positive outcomes across a large provider group. This is not an exhaustive list, but rather interventions that have high therapeutic yield.
- Identify, Treat, and Support Comorbidities
The above section, denoting the use of the “Klok” scale and assessment and therapeutic ideas, covers this area well.
Please note: If the comorbidities are not addressed, the therapeutics that follow will have partial or temporary effect at best. The therapeutics below assume underlying conditions and comorbidities are being addressed.
- Support Clotting / Coagulation Issues
Coagulopathies are ubiquitous in LC and PV. Treatment ranges from therapies for obvious coagulopathy to empiric treatment for microclotting. The focus therapeutically in the prescription realm is factor Xa drugs, heparin therapy, aspirin, clopidogrel, curcumin, and others. In the natural therapy realm, enzymes, essential fatty acids, vitamin E, and others are common.
A common clinical question is “Can people on factor Xa drugs, heparin therapy, aspirin, clopidogrel, or a combination of these use any natural anticoagulants?” The answer is generally yes, because the natural agents below work in different parts of the clotting cascade.
The following interventions are commonly used:
- Enzyme therapy is used in all patients. [I will use Nattokinase as an example, but Lumbrokinase or Serratiopeptidase (also known as serrapeptase) can work as well. Also note that Lumbrokinase may have more effect if thrombi have already formed.]112,113
- Nattokinase baseline dose is 2000 Units in the AM before eating
- Nattokinase in higher-risk people can be dosed between 4000 and 10,000 Units daily.
- Vitamin E as mixed tocopherols 200 to 400 IU daily
- Omega-3 1000 to 2000 mg total Omega-3 daily114-116
- Curcumin is a weak anticoagulant but an excellent tissue protectant. If it is clinically appropriate.102-104
- Different forms of curcumin have different dose strategies. I use Curcumin phytosome at 500 to 1000 mg per day
- In high-risk people, I will add 81 mg Aspirin daily (if they are not on it).134
- Bind Spike-protein and or Block from entering cells
Ivermectin 84 – 88, as well as quercetin40,107 – 111, curcumin102 – 104, rutin105, dandelion leaf135, milk thistle106, and Andrographis136 – 138 are studied for this.
My practice has been to use Ivermectin at 0.2 to 0.4 mg/kg (rounded to the nearest even number for prescribing ease) given in one dose at night, as it can have some GABA / somnolent effect. I do this daily for two to four months as a base therapy and then see how they do if I stop the dose. If they aggravate when discontinuing the Ivermectin, then they can return to the daily dose. It can be dosed long-term until the person has clinical remission. Safety note: I have used Ivermectin in cancer patients for over twenty years, and it is incredibly safe if used long-term.
I will generally add one more natural agent. If they are on curcumin, that is a good synergist. I typically add milk thistle 500 – 800 mg with dinner for this purpose, but also to help as a liver support as well. If “pill count overload” is an issue, then curcumin can fill that dual role.
Additionally, the flavonoid Rutin has some data on Spike-protein binding.105
- Degrade Spike-protein
Nattokinase is a subtilisin-like alkaline serine protease which has been shown not only to be anticoagulant112 but also to degrade the spike protein.113 Other enzymes in this class likely have similar effects, but nattokinase is the one currently with positive data. The above-mentioned dosing for anticoagulation can be sufficient. In sick individuals with high Spike antibodies, I will generally ensure the dose of nattokinase is 4000 – 10,000 Units daily away from food for the first three or more months.
There is a current discussion of the possibility of enzyme therapies aggravating these cases due to “incomplete breakdown” of the spike protein. While this theory sounds intriguing, if one looks at the data on how completely nattokinase breaks down the spike protein, it would appear this fear is unfounded.
- Depuration and Detoxification
Depuration (whole body supported elimination) and detoxification (specific pathway support) obviously go hand in hand. The process of LC or PV illness treatment will either directly or indirectly involve these factors.
If a comorbidity of a toxin / toxicant presence is found, then whatever specific therapies are used for that comorbidity may be enough to assist. With or without direct toxin / toxicant treatment, every person dealing with LC or PV has a need for some level of depuration and detoxification.
Options (as clinically indicated) include:
- Body movement and hydration
- Heat therapies
- Hydrotherapy of many types
- High fiber foods and or binder-type supplements
- Nutrient and antioxidant support
- Specific therapies (chelation, oxidation, etc.)
- Blood filtration therapies:
- Apheresis – Plasmapheresis / Therapeutic Plasma Exchange
- Extra-corporeal Blood Ozonation and Oxygenation (EBOO)
- Plasma donation
- Blood donation
- Modulate Immune Function
Both LC and PV are hallmarked by widespread immune deregulation. Some level of immune modulation support is necessary as treatment progresses. If comorbid infections exist, then this may be a longer process. Many agents already mentioned above have immunomodulatory activity (such as curcumin, Ivermectin, milk thistle, etc.), but some additions I have seen helpful are:
- Low Dose Naltrexone (LDN) dosed as in autoimmune cases93-97
- Acute H-1 and H-2 blockers (MCAS comorbidity)89 – 91
- Colchicine 0.5 or 0.6 mg BID for widespread inflammation, especially if vascular or cardiac comorbidity exists.97 – 99
- Melatonin 101 Melatonin has the effect of circadian support at lower doses (1 – 10 mg) and also can act as a mitochondrial redox support at higher (20 + mg) doses as well.
- A combination of Licorice 60 mg and Boswellia 200 mg given twice a day for fourteen days was also helpful.125
- Individually, Licorice120-124 and Boswellia127 have data as well.
- Optimizing Vitamin D levels
- Other studied agents include:
- Quercetin 40,107-111
- Luteolin117-119
- Rutin105
- Probiotics131-134
- And Cinnamon, Clove, Ginger, Garlic, Thyme, Cardamom139
- Support Antioxidant and Nutrient Status
I will not elaborate greatly on the need for support of basic nutrition and antioxidant levels in the LC and PV patient. It is well documented in 139 and other resources for anyone needing a reference. There is an excessive use of glutathione and vitamin C as water-soluble antioxidants in all chronic illnesses that should be addressed as the case progresses.
- Support Mitochondrial Function
Clinicians generally have their favorite “go-to” mitochondrial supports already in use. If this area is not addressed in patients with LC or PV, the case will typically progress slowly, and the patient’s quality of life will be low.
Always ensure that the basic factors that normally support the mitochondria and their function are in place. Dietary and supplemental amino acids, trace minerals, iron, B-vitamins, and other basic nutrients are crucial.
Acutely, the following have been useful in recovering energy and also supporting other healing pathways. These should be used within their typical clinical guidelines for dose and safety. A short, but commonly helpful, list is:
- ALA, Co-Q10, and other “mitochondrial support” combinations
- NAD Support, which can include IV NAD or NR, oral Niacinamide, and NR / NMN.
- Fasting130
- Methylene Blue oral and or IV
- Red – Near IR Photodynamic Therapy
- FIR Sauna
- PEMF
- Hyperbaric Oxygen Therapy
Healing Expectations:
In my experience, it is similar to most complex chronic illnesses as far as the length of time and complexity of care required. These cases of LC and PV can be some of the most difficult a provider will see. It does help both the clinician and patient to have a baseline “grading” using the Klok scale, as this can guide intensity of assessment and therapies, as well as prognosticate the healing trajectory.
Estimated time to work on healing is:
- Klok – 0:
This is more of a monitoring and prevention stage. If one finds a comorbidity on initial testing, then that may extend the time, but generally, this is an initial assessment, basic interventions, and follow-up as required.
- Klok 1 & 2:
Depending on the comorbidities found, these cases can start to see some progress in weeks to months. Generally, progress may wax and wane, but will be on an upward trajectory. Some patients feel remarkably improved in six months, and some in two years. As for following laboratory values, use typical follow-up intervals and let the patient know why you repeat some labs more or less frequently.
- Klok 3 & 4:
These patients typically have multiple comorbidities and require long-term care and attention. Some features may clear moderately quickly (an example being a thyroid condition found upon initial assessment). Other features, especially aggravated or new autoimmunity, toxicity, etc., may be very long-term issues. In many cases, these long-term issues are more chronic management items with the goal of improving overall function and quality of life rather than achieving a “full cure”.
- Note regarding using the Spike Antibody as baseline mentioned above: I generally do not test that more frequently than every six months. Symptomatic improvement is more critical than Spike Antibody drop. One correlation, however, to persistently elevated Spike Antibodies is the need to continue the Spike blocking, degrading, and anticoagulation therapies (as noted above) regardless of symptom improvement.
Factors that delay the healing trajectory:
- Things that “didn’t look like they needed to be checked”:
- Some common ones include rT3, Cortisol, reproductive hormones, toxin-toxicants, including chemical, metal, and mycotoxins. Autoimmunity and Infections, especially if not present prior.
- Not re-checking and adjusting acute use therapies:
- Endocrine, GI, Infectious, Toxic, etc.
- Generally, not following up on and treating pertinent positives from old labs or new findings.
- Not appreciating the impact of long-term production of Spike from the mRNA vaccines, or the impact of COVID reinfection.

Dr. Anderson is a recognized educator and clinician in integrative and naturopathic medicine with a focus on complex chronic illness and cancer. In addition to three decades of clinical experience, he was head of the interventional arm of a US-NIH-funded human research trial using IV and integrative therapies in cancer patients. He founded Advanced Medical Therapies in Seattle, Washington, a clinic focusing on cancer and chronic diseases. He now collaborates with clinics and hospitals in the US and other countries. His former positions included multiple medical school posts, as well as being a professor of pharmacology and clinical medicine at Bastyr University and chief of IV services for Bastyr Oncology Research Center.
References
- Anderson PS (2020) Intravenous ascorbic acid for supportive treatment in hospitalized COVID-19 patients. J Orthomol Med. 35(1)
- Anderson PS (2021) Update: Vitamin C in COVID-19. J Orthomol Med. 36(1)
- Chippa V, Aleem A, Anjum F. Postacute Coronavirus (COVID-19) Syndrome. 2024 Mar 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 34033370.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Global Health; Board on Health Sciences Policy; Committee on Examining the Working Definition for Long COVID. A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences. Goldowitz I, Worku T, Brown L, Fineberg HV, editors. Washington (DC): National Academies Press (US); 2024 Jul 9. PMID: 39110819.
- Halma, Matthew & Varon, Joseph. (2025). Breaking the silence: Recognizing post-vaccination syndrome. Heliyon. 11. e43478. 10.1016/j.heliyon.2025.e43478.
- Rahmati, M., Udeh, R., Kang, J., Dolja-Gore, X., McEvoy, M., Kazemi, A., Soysal, P., Smith, L., Kenna, T., Fond, G., Boussat, B., Nguyen, D.C., Do, H., Tran, B.X., Veronese, N., Yon, D.K. and Boyer, L. (2025), Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of Symptoms 3 Years Post-SARS-CoV-2 Infection. Journal of Medical Virology, 97: e70429. https://doi.org/10.1002/jmv.70429
- Posa A. Spike protein-related proteinopathies: A focus on the neurological side of spikeopathies. Ann Anat. 2025 Jun;260:152662. doi: 10.1016/j.aanat.2025.152662. Epub 2025 Apr 18. PMID: 40254264.
- Li Y, Li J, Dang Y, Chen Y, Tao C. Adverse Events of COVID-19 Vaccines in the United States: Temporal and Spatial Analysis. JMIR Public Health Surveill. 2024 Jul 15;10:e51007. doi: 10.2196/51007. PMID: 39008362; PMCID: PMC11287098.
- Yaamika H, Muralidas D, Elumalai K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J Taibah Univ Med Sci. 2023 Sep 5;18(6):1646-1661. doi: 10.1016/j.jtumed.2023.08.004. PMID: 37732332; PMCID: PMC10507236.
- Seo JW, Kim SE, Kim Y, Kim EJ, Kim T, Kim T, Lee SH, Lee E, Lee J, Seo YB, Jeong YH, Jung YH, Choi YJ, Song JY. Updated Clinical Practice Guidelines for the Diagnosis and Management of Long COVID. Infect Chemother. 2024 Mar;56(1):122-157. doi: 10.3947/ic.2024.0024. Epub 2024 Mar 13. PMID: 38527781; PMCID: PMC10990882.
- Greenhalgh, Trisha et al. Long COVID: a clinical update. The Lancet, Volume 404, Issue 10453, 707 – 724
- Ahmed N, Mahmood MS, Ullah MA, Araf Y, Rahaman TI, Moin AT, Hosen MJ. COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr Microbiol. 2022 Mar 14;79(5):127. doi: 10.1007/s00284-022-02824-6. PMID: 35287179; PMCID: PMC8918595.
- Zhu X, Li Y, Wang J, Gao W. Clinical Features of Long COVID Patients Coinfected With Mycoplasma pneumoniae. Can J Infect Dis Med Microbiol. 2024 Dec 7;2024:7213129. doi: 10.1155/cjid/7213129. PMID: 39679212; PMCID: PMC11646145.
- Nicolson, G. and de Mattos, G. (2020) COVID-19 Coronavirus: Is Infection along with Mycoplasma or Other Bacteria Linked to Progression to a Lethal Outcome?. International Journal of Clinical Medicine, 11, 282-302. doi: 10.4236/ijcm.2020.115029.
- Bernal KDE, Whitehurst CB. Incidence of Epstein-Barr virus reactivation is elevated in COVID-19 patients. Virus Res. 2023 Sep;334:199157. doi: 10.1016/j.virusres.2023.199157. Epub 2023 Jun 26. PMID: 37364815; PMCID: PMC10292739.
- Ahmed N, Mahmood MS, Ullah MA, Araf Y, Rahaman TI, Moin AT, Hosen MJ. COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr Microbiol. 2022 Mar 14;79(5):127. doi: 10.1007/s00284-022-02824-6. PMID: 35287179; PMCID: PMC8918595.
- Ach T, Ben Haj Slama N, Gorchane A, Ben Abdelkrim A, Garma M, Ben Lasfar N, Bellazreg F, Debbabi W, Hachfi W, Chadli Chaieb M, Zaouali M, Letaief A, Ach K. Explaining Long COVID: A Pioneer Cross-Sectional Study Supporting the Endocrine Hypothesis. J Endocr Soc. 2024 Jan 11;8(3):bvae003. doi: 10.1210/jendso/bvae003. PMID: 38260089; PMCID: PMC10801829.
- Koch CA, Long Covid: Hormone Imbalances and/or Rather Complex Immune Dysregulations?, Journal of the Endocrine Society, Volume 8, Issue 5, May 2024, bvae043, https://doi.org/10.1210/jendso/bvae043
- El-Rhermoul FZ, Fedorowski A, Eardley P, Taraborrelli P, Panagopoulos D, Sutton R, Lim PB, Dani M. Autoimmunity in Long Covid and POTS. Oxf Open Immunol. 2023 Mar 8;4(1):iqad002. doi: 10.1093/oxfimm/iqad002. PMID: 37255928; PMCID: PMC10224806.
- Bhattacharjee B, et.al. Immunological and Antigenic Signatures Associated with Chronic Illnesses after COVID-19 Vaccination medRxiv 2025.02.18.25322379; doi: https://doi.org/10.1101/2025.02.18.25322379
- Guedes de Sa KS, Silva J. Et.al. A causal link between autoantibodies and neurological symptoms in long COVID. medRxiv 2024.06.18.24309100; doi: https://doi.org/10.1101/2024.06.18.24309100
- Huang SF, Ying-Jung Wu A, Shin-Jung Lee S, Huang YS, Lee CY, Yang TL, Wang HW, Chen HJ, Chen YC, Ho TS, Kuo CF, Lin YT; GREAT working group. COVID-19 associated mold infections: Review of COVID-19 associated pulmonary aspergillosis and mucormycosis. J Microbiol Immunol Infect. 2023 Jun;56(3):442-454. doi: 10.1016/j.jmii.2022.12.004. Epub 2022 Dec 15. PMID: 36586744; PMCID: PMC9751001.
- Koulenti D, Paramythiotou E, Almyroudi MP, Karvouniaris M, Markou N, Paranos P, Routsi C, Meletiadis J, Blot S. Severe mold fungal infections in critically ill patients with COVID-19. Future Microbiol. 2024 Jun 12;19(9):825-840. doi: 10.2217/fmb-2023-0261. Epub 2024 May 31. PMID: 38700287; PMCID: PMC11290760.
- Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020 Oct;26(10):1395-1399. doi: 10.1016/j.cmi.2020.06.025. Epub 2020 Jun 27. PMID: 32603803; PMCID: PMC7320692.
- Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, Zhu F, Zhu B, Cui L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020 Aug;285:198005. doi: 10.1016/j.virusres.2020.198005. Epub 2020 May 11. PMID: 32408156; PMCID: PMC7213959.
- D’Abramo A, Lepore L, Palazzolo C, Barreca F, Liuzzi G, Lalle E, Nicastri E. Acute respiratory distress syndrome due to SARS-CoV-2 and Influenza A co-infection in an Italian patient: Mini-review of the literature. Int J Infect Dis. 2020 Aug;97:236-239. doi: 10.1016/j.ijid.2020.06.056. Epub 2020 Jun 18. PMID: 32565366; PMCID: PMC7301795.
- Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, Cornely OA, S Perlin D, Lass-Flörl C, Hoenigl M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)-From Immunology to Treatment. J Fungi (Basel). 2020 Jun 24;6(2):91. doi: 10.3390/jof6020091. PMID: 32599813; PMCID: PMC7346000.
- Lv Z, Cheng S, Le J, Huang J, Feng L, Zhang B, Li Y. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. 2020 May-Jun;22(4-5):195-199. doi: 10.1016/j.micinf.2020.05.007. Epub 2020 May 18. PMID: 32425649; PMCID: PMC7233257.
- Wee LE, Ko KKK, Ho WQ, Kwek GTC, Tan TT, Wijaya L. Community-acquired viral respiratory infections amongst hospitalized inpatients during a COVID-19 outbreak in Singapore: co-infection and clinical outcomes. J Clin Virol. 2020 Jul;128:104436. doi: 10.1016/j.jcv.2020.104436. Epub 2020 May 19. PMID: 32447256; PMCID: PMC7235565.
- Baalbaki N, Slob EMA, Kazer SW, I Abdel-Aziz M, Bogaard HJ, Golebski K, Maitland-van der Zee AH. The Omics Landscape of Long COVID-A Comprehensive Systematic Review to Advance Biomarker, Target and Drug Discovery. Allergy. 2025 Apr;80(4):932-948. doi: 10.1111/all.16526. Epub 2025 Mar 14. PMID: 40084919; PMCID: PMC11969314.
- Lammi, V., Nakanishi, T., Jones, S.E. et al. Genome-wide association study of long COVID. Nat Genet 57, 1402–1417 (2025). https://doi.org/10.1038/s41588-025-02100-w
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023 Mar;21(3):133-146. doi: 10.1038/s41579-022-00846-2. Epub 2023 Jan 13. Erratum in: Nat Rev Microbiol. 2023 Jun;21(6):408. doi: 10.1038/s41579-023-00896-0. PMID: 36639608; PMCID: PMC9839201.
- Theoharides TC, Conti P. Be aware of SARS-CoV-2 spike protein: There is more than meets the eye. J Biol Regul Homeost Agents. 2021 May-Jun;35(3):833-838. doi: 10.23812/THEO_EDIT_3_21. PMID: 34100279.
- Montone RA, Rinaldi R, Masciocchi C, Lilli L, Damiani A, La Vecchia G, Iannaccone G, Basile M, Salzillo C, Caffè A, Bonanni A, De Pascale G, Grieco DL, Tanzarella ES, Buonsenso D, Murri R, Fantoni M, Liuzzo G, Sanna T, Richeldi L, Sanguinetti M, Massetti M, Trani C, Tshomba Y, Gasbarrini A, Valentini V, Antonelli M, Crea F. Vaccines and myocardial injury in patients hospitalized for COVID-19 infection: the CardioCOVID-Gemelli study. Eur Heart J Qual Care Clin Outcomes. 2025 Jan 16;11(1):59-67. doi: 10.1093/ehjqcco/qcae016. PMID: 38414273; PMCID: PMC11736151.
- Parry PI, Lefringhausen A, Turni C, Neil CJ, Cosford R, Hudson NJ, Gillespie J. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines. 2023 Aug 17;11(8):2287. doi: 10.3390/biomedicines11082287. PMID: 37626783; PMCID: PMC10452662.
- Tsilioni I, Theoharides TC. Recombinant SARS-CoV-2 Spike Protein and Its Receptor Binding Domain Stimulate Release of Different Pro-Inflammatory Mediators via Activation of Distinct Receptors on Human Microglia Cells. Mol Neurobiol. 2023 Nov;60(11):6704-6714. doi: 10.1007/s12035-023-03493-7. Epub 2023 Jul 21. PMID: 37477768.
- Antonopoulou S, Petsini F, Detopoulou M, Theoharides TC, Demopoulos CA. Is there an interplay between the SARS-CoV-2 spike protein and Platelet-Activating factor? Biofactors. 2022 Nov;48(6):1271-1283. doi: 10.1002/biof.1877. Epub 2022 Jul 19. PMID: 35852257; PMCID: PMC9349578.
- Tsilioni I, Theoharides TC. Recombinant SARS-CoV-2 Spike Protein Stimulates Secretion of Chymase, Tryptase, and IL-1β from Human Mast Cells, Augmented by IL-33. Int J Mol Sci. 2023 May 30;24(11):9487. doi: 10.3390/ijms24119487. PMID: 37298438; PMCID: PMC10253625.
- Theoharides, T.C.; Kempuraj, D. Role of SARS-CoV-2 Spike-Protein-Induced Activation of Microglia and Mast Cells in the Pathogenesis of Neuro-COVID. Cells 2023, 12, 688. https://doi.org/ 10.3390/cells12050688
- Theoharides TC. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol Neurobiol. 2022 Mar;59(3):1850-1861. doi: 10.1007/s12035-021-02696-0. Epub 2022 Jan 13. PMID: 35028901; PMCID: PMC8757925.
- Persel C, Ashley M, MD, JD. COVID-19 and Brain Injury. November 3, 2020. https://www.psychiatrictimes.com/view/covid-19-brain-injury
- Boldrini M, Canoll PD, Klein RS. How COVID-19 Affects the Brain. JAMA Psychiatry. 2021;78(6):682–683. doi:10.1001/jamapsychiatry.2021.0500
- Buonsenso, Danilo et al. Long COVID and SARS-CoV-2 persistence: new answers, more questions. The Lancet Infectious Diseases, Volume 24, Issue 8, 796 – 798
- Boros LG, Kyriakopoulos AM, Brogna C, Piscopo M, McCullough PA, Seneff S. Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol Res Perspect. 2024 Jun;12(3):e1218. doi: 10.1002/prp2.1218. PMID: 38867495; PMCID: PMC11169277.
- Jahankhani K, Ahangari F, Adcock IM, Mortaz E. Possible cancer-causing capacity of COVID-19: Is SARS-CoV-2 an oncogenic agent? Biochimie. 2023 Oct;213:130-138. doi: 10.1016/j.biochi.2023.05.014. Epub 2023 May 23. PMID: 37230238; PMCID: PMC10202899.
- Kim MJ, Kim JY, Shin JH, Son J, Kang Y, Jeong SK, Kim DH, Kim KH, Chun E, Lee KY. The SARS-CoV-2 spike protein induces lung cancer migration and invasion in a TLR2-dependent manner. Cancer Commun (Lond). 2024 Feb;44(2):273-277. doi: 10.1002/cac2.12485. Epub 2023 Sep 13. PMID: 37702496; PMCID: PMC10876188.
- Li YS, Ren HC, Cao JH. Correlation of SARS‑CoV‑2 to cancer: Carcinogenic or anticancer? (Review). Int J Oncol. 2022 Apr;60(4):42. doi: 10.3892/ijo.2022.5332. Epub 2022 Mar 2. PMID: 35234272; PMCID: PMC8923649
- Parry PI, Lefringhausen A, Turni C, Neil CJ, Cosford R, Hudson NJ, Gillespie J. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines. 2023 Aug 17;11(8):2287. doi: 10.3390/biomedicines11082287. PMID: 37626783; PMCID: PMC10452662.
- Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 2022 Jun;164:113008. doi: 10.1016/j.fct.2022.113008. Epub 2022 Apr 15. PMID: 35436552; PMCID: PMC9012513.
- Singh N, Bharara Singh A. S2 subunit of SARS-nCoV-2 interacts with tumor suppressor protein p53 and BRCA: an in silico study. Transl Oncol. 2020 Oct;13(10):100814. doi: 10.1016/j.tranon.2020.100814. Epub 2020 Jun 30. PMID: 32619819; PMCID: PMC7324311.
- Valdes Angues R, Perea Bustos Y. SARS-CoV-2 Vaccination and the Multi-Hit Hypothesis of Oncogenesis. Cureus. 2023 Dec 17;15(12):e50703. doi: 10.7759/cureus.50703. PMID: 38234925; PMCID: PMC10792266.
- Zhang S, El-Deiry WS. Transfected SARS-CoV-2 spike DNA for mammalian cell expression inhibits p53 activation of p21(WAF1), TRAIL Death Receptor DR5 and MDM2 proteins in cancer cells and increases cancer cell viability after chemotherapy exposure. Oncotarget. 2024 May 3;15:275-284. doi: 10.18632/oncotarget.28582. PMID: 38709242; PMCID: PMC11073320.
- Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer. 2021 May 17;20(1):76. doi: 10.1186/s12943-021-01363-1. PMID: 34001144; PMCID: PMC8126512.
- Favaloro EJ. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int J Lab Hematol. 2021 Aug;43(4):559-570. doi: 10.1111/ijlh.13629. Epub 2021 Jun 17. PMID: 34138513; PMCID: PMC8444734.
- Yazdani AN, DeMarco N, Patel P, Abdi A, Velpuri P, Agrawal DK, Rai V. Adverse Hematological Effects of COVID-19 Vaccination and Pathomechanisms of Low Acquired Immunity in Patients with Hematological Malignancies. Vaccines (Basel). 2023 Mar 15;11(3):662. doi: 10.3390/vaccines11030662. PMID: 36992246; PMCID: PMC10058097.
- Dalton CF, de Oliveira MIR, Stafford P, et al. Increased fibrinoid microclot counts in platelet-poor plasma are associated with Long COVID. medRxiv. 2024. https://doi.org/10.1101/2024.04.04.24305318
- Okazaki E, Barion BG, da Rocha TRF, et al. Persistent hypofibrinolysis in severe COVID-19 associated with elevated fibrinolysis inhibitors activity. J Thromb Thrombolysis. 2024;57:721-729. https://doi.org/10.1007/s11239-024-02961-8
- Grobbelaar LM, Venter C, Vlok M, et al. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. medRxiv. 2021. https://doi.org/10.1101/2021.03.05.21252960
- Turner S, Naidoo CA, Usher TJ, et al. Increased levels of inflammatory molecules in blood of Long COVID patients point to thrombotic endotheliitis. medRxiv. 2022. https://doi.org/10.1101/2022.10.13.22281055
- Hranjec T, Estreicher M, Rogers B, et al. Integral Use of Thromboelastography With Platelet Mapping to Guide Appropriate Treatment, Avoid Complications, and Improve Survival of Patients With Coronavirus Disease 2019-Related Coagulopathy. Crit Care Explor. 2020;2(12):e0287.
- Pretorius E, Venter C, Laubscher GJ, et al. Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: a preliminary report. Cardiovasc Diabetol. 2020;19(1):193.
- Meizoso JP, Moore HB, Moore EE. Fibrinolysis Shutdown in COVID-19: Clinical Manifestations, Molecular Mechanisms, and Therapeutic Implications. J Am Coll Surg. 2021;232(6):995-1003.
- Akhtar M, Islam MR, Khaton F, Rahman F, Sami TA, Tauheed I, Ahmed T, Akter A, Khan II, Khan ZH, Kumar Biswas P, Ryan ET, Banu S, Shirin T, Chowdhury F, Khan AI, Bhuiyan TR, Qadri F. Spike specific IgG3 and nucleocapsid IgG response in serum serve as distinguishing immunological markers between SARS-CoV-2 infection and vaccination. Front Immunol. 2025 Mar 27;16:1518915. doi: 10.3389/fimmu.2025.1518915. PMID: 40213555; PMCID: PMC11983542.
- Koenig A, Quasem M and Zink B et al. The Role of SARS-CoV-2 Nucleocapsid Protein Persistence in Inducing Chronic Type I Interferon and Mitochondrial Dysfunction. 2025. DOI: 10.1101/2025.06.02.657400
- Swadźba J, Panek A, Wąsowicz P, Anyszek T, Martin E. High Concentration of Anti-SARS-CoV-2 Antibodies 2 Years after COVID-19 Vaccination Stems Not Only from Boosters but Also from Widespread, Often Unrecognized, Contact with the Virus. Vaccines (Basel). 2024 Apr 28;12(5):471. doi: 10.3390/vaccines12050471. PMID: 38793722; PMCID: PMC11125768.
- Poore B, Nerenz RD, Brodis D, Brown CI, Cervinski MA, Hubbard JA. A comparison of SARS-CoV-2 nucleocapsid and spike antibody detection using three commercially available automated immunoassays. Clin Biochem. 2021 Sep;95:77-80. doi: 10.1016/j.clinbiochem.2021.05.011. Epub 2021 Jun 9. PMID: 34118242; PMCID: PMC8188801.
- Patterson BK, Yogendra R, Francisco EB, Guevara-Coto J, Long E, Pise A, Osgood E, Bream J, Kreimer M, Jeffers D, Beaty C, Vander Heide R, Mora-Rodríguez RA. Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals. Hum Vaccin Immunother. 2025 Dec;21(1):2494934. doi: 10.1080/21645515.2025.2494934. Epub 2025 May 13. PMID: 40358138; PMCID: PMC12077440.
- Uversky VN, Redwan EM, Makis W, Rubio-Casillas A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines (Basel). 2023 May 17;11(5):991. doi: 10.3390/vaccines11050991. PMID: 37243095; PMCID: PMC10222767.
- Yazdani AN, DeMarco N, Patel P, Abdi A, Velpuri P, Agrawal DK, Rai V. Adverse Hematological Effects of COVID-19 Vaccination and Pathomechanisms of Low Acquired Immunity in Patients with Hematological Malignancies. Vaccines (Basel). 2023 Mar 15;11(3):662. doi: 10.3390/vaccines11030662. PMID: 36992246; PMCID: PMC10058097.
- Hulscher N, Alexander P E., Amerling R, Gessling H, Hodkinson R, Makis W et al. A Systematic Review Of Autopsy Findings In Deaths After COVID-19 Vaccination. Science, Public Health Policy and the Law. 2024 Nov 17; v5.2019-2024
- https://inmodia.de/en/
- Low RN, Low RJ, Akrami A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front Med (Lausanne). 2023 Mar 31;10:1011936. doi: 10.3389/fmed.2023.1011936. PMID: 37064029; PMCID: PMC10103649.
- Kong M, Zhang H, Cao X, Mao X, Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol Infect. 2020 Jul 9;148:e139. doi: 10.1017/S0950268820001557. PMID: 32641174; PMCID: PMC7360950.
- https://www.consultdranderson.com/nhanes-2015-updates-from-the-fourth-report/
- Klein, J., Wood, J., Jaycox, J.R. et al. Distinguishing features of long COVID identified through immune profiling. Nature 623, 139–148 (2023). https://doi.org/10.1038/s41586-023-06651-y
- Nicolson, G. and de Mattos, G. (2020) COVID-19 Coronavirus: Is Infection along with Mycoplasma or Other Bacteria Linked to Progression to a Lethal Outcome?. International Journal of Clinical Medicine, 11, 282-302. doi: 10.4236/ijcm.2020.115029.
- Klein, J., Wood, J., Jaycox, J.R. et al. Distinguishing features of long COVID identified through immune profiling. Nature 623, 139–148 (2023). https://doi.org/10.1038/s41586-023-06651-y
- Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, Davis JP, Loiselle M, Novak T, Senussi Y, Cheng CA, Burgess E, Edlow AG, Chou J, Dionne A, Balaguru D, Lahoud-Rahme M, Arditi M, Julg B, Randolph AG, Alter G, Fasano A, Walt DR. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023 Mar 14;147(11):867-876. doi: 10.1161/CIRCULATIONAHA.122.061025. Epub 2023 Jan 4. PMID: 36597886; PMCID: PMC10010667.
- Sutton WJH, Branham PJ, Williamson YM, Cooper HC, Najjar FN, Pierce-Ruiz CL, Barr JR, Williams TL. Quantification of SARS-CoV-2 spike protein expression from mRNA vaccines using isotope dilution mass spectrometry. Vaccine. 2023 Jun 13;41(26):3872-3884. doi: 10.1016/j.vaccine.2023.04.044. Epub 2023 May 8. PMID: 37202272; PMCID: PMC10165024.
- Li YS, Ren HC, Cao JH. Correlation of SARS‑CoV‑2 to cancer: Carcinogenic or anticancer? (Review). Int J Oncol. 2022 Apr;60(4):42. doi: 10.3892/ijo.2022.5332. Epub 2022 Mar 2. PMID: 35234272; PMCID: PMC8923649
- Patterson BK, Guevara-Coto J, Yogendra R, Francisco EB, Long E, Pise A, Rodrigues H, Parikh P, Mora J, Mora-Rodríguez RA. Immune-Based Prediction of COVID-19 Severity and Chronicity Decoded Using Machine Learning. Front Immunol. 2021 Jun 28;12:700782. doi: 10.3389/fimmu.2021.700782. PMID: 34262570; PMCID: PMC8273732.
- https://www.consultdranderson.com/anderson-biofilm-phase-2-bt-handout/
- Tanikawa T, Kiba Y, Yu J, Hsu K, Chen S, Ishii A, Yokogawa T, Suzuki R, Inoue Y, Kitamura M. Degradative Effect of Nattokinase on Spike Protein of SARS-CoV-2. Molecules. 2022 Aug 24;27(17):5405. doi: 10.3390/molecules27175405. PMID: 36080170; PMCID: PMC9458005.
- Behera, P.; Patro, B.K.; Singh, A.K.; Chandanshive, P.D.; Ravikumar, S.R.; Pradhan, S.K.; Pentapati, S.S.K.; Batmanabane, G.;Mohapatra, P.R.; Padhy, B.M.; et al. Role of Ivermectin in the Prevention of SARS-CoV-2 Infection among Healthcare Workers in India: A Matched Case-Control Study. PLoS ONE 2021, 16, e0247163.
- Zaidi, A.K.; Dehgani-Mobaraki, P. The Mechanisms of Action of Ivermectin against SARS-CoV-2—An Extensive Review. J. Antibiot. 2022, 75, 60–71. [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.;Wagstaff, K.M. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro. Antivir. Res. 2020, 178, 104787. [CrossRef]
- Bryant, A.; Lawrie, T.A.; Dowswell, T.; Fordham, E.J.; Mitchell, S.; Hill, S.R.; Tham, T.C. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am. J.Ther. 2021, 28, e434–e460. [CrossRef]
- Kory, P.; Meduri, G.U.; Varon, J.; Iglesias, J.; Marik, P.E. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. Am. J. Ther. 2021, 28, e299–e318.
- Morán Blanco, J.I.; Alvarenga Bonilla, J.A.; Homma, S.; Suzuki, K.; Fremont-Smith, P.; Villar Gómez de las Heras, K. Antihistamines and Azithromycin as a Treatment for COVID-19 on Primary Health Care—A Retrospective Observational Study in Elderly Patients. Pulm. Pharmacol. Ther. 2021, 67, 101989.
- Pinto, M.D.; Lambert, N.; Downs, C.A.; Abrahim, H.; Hughes, T.D.; Rahmani, A.M.; Burton, C.W.; Chakraborty, R. Antihistamines for Postacute Sequelae of SARS-CoV-2 Infection. J. Nurse Pract. 2022, 18, 335–338.
- Reznikov, L.R.; Norris, M.H.; Vashisht, R.; Bluhm, A.P.; Li, D.; Liao, Y.-S.J.; Brown, A.; Butte, A.J.; Ostrov, D.A. Identification of Antiviral Antihistamines for COVID-19 Repurposing. Biochem. Biophys. Res. Commun. 2021, 538, 173–179.
- Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Further Evidence for the Use of Aspirin in COVID-19. Int. J. Cardiol. 2022, 346, 107–108.
- Naik H, Cooke E, Boulter T, et alLow-dose naltrexone for post-COVID fatigue syndrome: a study protocol for a double-blind, randomised trial in British Columbia BMJ Open 2024;14:e085272. doi: 10.1136/bmjopen-2024-085272
- Tamariz L, Bast E, Klimas N, Palacio A. Low-dose Naltrexone Improves post-COVID-19 condition Symptoms. Clin Ther. 2024 Mar;46(3):e101-e106. doi: 10.1016/j.clinthera.2023.12.009. Epub 2024 Jan 23. PMID: 38267326.
- Choubey, A.; Dehury, B.; Kumar, S.; Medhi, B.; Mondal, P. Naltrexone a Potential Therapeutic Candidate for COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 963–970.
- O’Kelly, B.; Vidal, L.; McHugh, T.;Woo, J.; Avramovic, G.; Lambert, J.S. Safety and Efficacy of Low Dose Naltrexone in a Long Covid Cohort; an Interventional Pre-Post Study. Brain. Behav. Immun. Health 2022, 24, 100485.
- Karatza, E.; Ismailos, G.; Karalis, V. Colchicine for the Treatment of COVID-19 Patients: Efficacy, Safety, and Model Informed Dosage Regimens. Xenobiotica 2021, 51, 643–656.
- Chiu, L.; Lo, C.-H.; Shen, M.; Chiu, N.; Aggarwal, R.; Lee, J.; Choi, Y.-G.; Lam, H.; Prsic, E.H.; Chow, R.; et al. Colchicine Use in Patients with COVID-19: A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0261358.
- Rabbani, A.B.; Parikh, R.V.; Rafique, A.M. Colchicine for the Treatment of Myocardial Injury in PatientsWith Coronavirus Disease 2019 (COVID-19)—An Old DrugWith New Life? JAMA Netw. Open 2020, 3, e2013556.
- Isman A, Nyquist A, Strecker B, Harinath G, Lee V, Zhang X, Zalzala S. Low-dose naltrexone and NAD+ for the treatment of patients with persistent fatigue symptoms after COVID-19. Brain Behav Immun Health. 2024 Feb 1;36:100733. doi: 10.1016/j.bbih.2024.100733. PMID: 38352659; PMCID: PMC10862402.
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Possible Application of Melatonin in Long COVID. Biomolecules 2022, 12, 1646.
- Jena, A.B.; Kanungo, N.; Nayak, V.; Chainy, G.B.N.; Dandapat, J. Catechin and Curcumin Interact with S Protein of SARS-CoV2 and ACE2 of Human Cell Membrane: Insights from Computational Studies. Sci. Rep. 2021, 11, 2043.
- Rattis, B.A.C.; Ramos, S.G.; Celes, M.R.N. Curcumin as a Potential Treatment for COVID-19. Front. Pharmacol. 2021, 12, 2085.
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.;Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256.
- Kumari, A.; Rajput, V.S.; Nagpal, P.; Kukrety, H.; Grover, S.; Grover, A. Dual Inhibition of SARS-CoV-2 Spike and Main Protease through a Repurposed Drug, Rutin. J. Biomol. Struct. Dyn. 2022, 40, 4987–4999.
- Speciale, A.; Muscarà, C.; Molonia, M.S.; Cimino, F.; Saija, A.; Giofrè, S.V. Silibinin as Potential Tool against SARS-Cov-2: In Silico Spike Receptor-Binding Domain and Main Protease Molecular Docking Analysis, and in Vitro Endothelial Protective Effects. Phytother. Res. 2021, 35, 4616–4625.
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A Role for Quercetin in Coronavirus Disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236.
- Tuli, H.; Sood, S.; Pundir, A.; Choudhary, D.; Dhama, K.; Kaur, G.; Seth, P.; Vashishth, A.; Kumar, P. Molecular Docking Studies of Apigenin, Kaempferol, and Quercetin as Potential Target against Spike Receptor Protein of SARS COV. J. Exp. Biol. Agric. Sci. 2022, 10, 144–149.
- Önal, H.; Arslan, B.; Üçüncü Ergun, N.; Topuz, ¸S.; Yilmaz Semerci, S.; Kurnaz, M.E.; Molu, Y.M.; Bozkurt, M.A.; Süner, N.; Kocata¸s,A. Treatment of COVID-19 Patients with Quercetin: A Prospective, Single Center, Randomized, Controlled Trial. Turk. J. Biol. 2021, 45, 518–529.
- Pan, B.; Fang, S.; Zhang, J.; Pan, Y.; Liu, H.; Wang, Y.; Li, M.; Liu, L. Chinese Herbal Compounds against SARS-CoV-2: Puerarin and Quercetin Impair the Binding of Viral S-Protein to ACE2 Receptor. Comput. Struct. Biotechnol. J. 2020, 18, 3518–3527.
- Manjunath, S.H.; Thimmulappa, R.K. Antiviral, Immunomodulatory, and Anticoagulant Effects of Quercetin and Its Derivatives: Potential Role in Prevention and Management of COVID-19. J. Pharm. Anal. 2022, 12, 29–34.
- Oba, M.; Rongduo, W.; Saito, A.; Okabayashi, T.; Yokota, T.; Yasuoka, J.; Sato, Y.; Nishifuji, K.;Wake, H.; Nibu, Y.; et al. Natto Extract, a Japanese Fermented Soybean Food, Directly Inhibits Viral Infections Including SARS-CoV-2 in Vitro. Biochem. Biophys. Res. Commun. 2021, 570, 21–25.
- Tanikawa T, Kiba Y, Yu J, Hsu K, Chen S, Ishii A, Yokogawa T, Suzuki R, Inoue Y, Kitamura M. Degradative Effect of Nattokinase on Spike Protein of SARS-CoV-2. Molecules. 2022 Aug 24;27(17):5405. doi: 10.3390/molecules27175405. PMID: 36080170; PMCID: PMC9458005.
- Ma, Y.; Zhang, L.; Zeng, R.; Luo, D.; Jiang, R.;Wu, H.; Zhuo, Z.; Yang, Q.; Li, J.; Leung, F.W.; et al. Associations of Habitual Fish Oil Use with Risk of SARS-CoV-2 Infection and COVID-19-Related Outcomes in UK: National Population Based Cohort Study. medRxiv 2022.
- Merritt, R.J.; Bhardwaj, V.; Jami, M.M. Fish Oil and COVID-19 Thromboses. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 1120.
- Torrinhas, R.S.; Calder, P.C.; Lemos, G.O.; Waitzberg, D.L. Parenteral Fish Oil: An Adjuvant Pharmacotherapy for Coronavirus Disease 2019? Nutrition 2021, 81, 110900.
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID Syndrome-associated Brain Fog and Chemofog: Luteolin to the Rescue. Biofactors 2021, 47, 232–241.
- Shadrack, D.M.; Deogratias, G.; Kiruri, L.W.; Onoka, I.; Vianney, J.-M.; Swai, H.; Nyandoro, S.S. Luteolin: A Blocker of SARS-CoV-2 Cell Entry Based on Relaxed Complex Scheme, Molecular Dynamics Simulation, and Metadynamics. J. Mol. Model 2021, 27, 221.
- Theoharides, T.C. COVID-19, Pulmonary Mast Cells, Cytokine Storms, and Beneficial Actions of Luteolin. Biofactors 2020, 46, 306–308.
- Gomaa, A.A.; Abdel-Wadood, Y.A. The Potential of Glycyrrhizin and Licorice Extract in Combating COVID-19 and Associated Conditions. Phytomed. Plus 2021, 1, 100043.
- van de Sand, L.; Bormann, M.; Alt, M.; Schipper, L.; Heilingloh, C.S.; Steinmann, E.; Todt, D.; Dittmer, U.; Elsner, C.; Witzke, O.; et al. Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses 2021, 13, 609.
- Diomede, L.; Beeg, M.; Gamba, A.; Fumagalli, O.; Gobbi, M.; Salmona, M. Can Antiviral Activity of Licorice Help Fight COVID-19 Infection? Biomolecules 2021, 11, 855.
- Gomaa, A. Evaluation of The Potential Therapeutic Effects of Licorice and Boswellia Serrata Gum in Egyptian Patients With COVID-19 as a Complementary Medicine. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04487964
- Ng, S.L.; Khaw, K.-Y.; Ong, Y.S.; Goh, H.P.; Kifli, N.; Teh, S.P.; Ming, L.C.; Kotra, V.; Goh, B.H. Licorice: A Potential Herb in Overcoming SARS-CoV-2 Infections. J. Evid. Based. Integr. Med. 2021, 26, 2515690X21996662.
- Gomaa AA, Mohamed HS, Abd-Ellatief RB, Gomaa MA, Hammam DS. Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial. Inflammopharmacology. 2022 Apr;30(2):477-486. doi: 10.1007/s10787-022-00939-7. Epub 2022 Mar 1. PMID: 35233748; PMCID: PMC8886861.
- Gomaa AA, Abdel-Wadood YA, Gomaa MA. Glycyrrhizin and boswellic acids, the golden nutraceuticals: multitargeting for treatment of mild-moderate COVID-19 and prevention of post-COVID cognitive impairment. Inflammopharmacology. 2022 Dec;30(6):1977-1992. doi: 10.1007/s10787-022-01062-3. Epub 2022 Sep 22. PMID: 36136251; PMCID: PMC9493173.
- Gaylis NB, Kreychman I, Sagliani J, Mograbi J, Gabet Y. The results of a unique dietary supplement (nutraceutical formulation) used to treat the symptoms of long-haul COVID. Front Nutr. 2022 Oct 25;9:1034169. doi: 10.3389/fnut.2022.1034169. PMID: 36386945; PMCID: PMC9641293.
- Zheng M, Schultz MB, Sinclair DA. NAD+ in COVID-19 and viral infections. Trends Immunol. 2022 Apr;43(4):283-295. doi: 10.1016/j.it.2022.02.001. Epub 2022 Feb 11. PMID: 35221228; PMCID: PMC8831132.
- Schreiber, S., Waetzig, G.H., López-Agudelo, V.A. et al. Nicotinamide modulates gut microbial metabolic potential and accelerates recovery in mild-to-moderate COVID-19. Nat Metab 7, 1136–1149 (2025). https://doi.org/10.1038/s42255-025-01290-1
- Dongoran RA, Mardiana M, Huang CY, Situmorang JH. Boosting NAD+ levels through fasting to aid in COVID-19 recovery. Front Immunol. 2024 Feb 14;15:1319106. doi: 10.3389/fimmu.2024.1319106. PMID: 38420124; PMCID: PMC10899445.
- Lim HX, Khalid K, Abdullah ADI, Lee LH, Raja Ali RA. Subphenotypes of Long COVID and the clinical applications of probiotics. Biomed Pharmacother. 2025 Feb;183:117855. doi: 10.1016/j.biopha.2025.117855. Epub 2025 Jan 24. PMID: 39862702.
- Docampo MJ, Batruch M, Oldrati P, Berenjeno-Correa E, Hilty M, Leventhal G, Lutterotti A, Martin R, Sospedra M. Clinical and Immunologic Effects of Paraprobiotics in Long-COVID Patients: A Pilot Study. Neurol Neuroimmunol Neuroinflamm. 2024 Sep;11(5):e200296. doi: 10.1212/NXI.0000000000200296. Epub 2024 Aug 6. PMID: 39106427; PMCID: PMC11318528.
- Alenazy MF, Aljohar HI, Alruwaili AR, Daghestani MH, Alonazi MA, Labban RS, El-Ansary AK, Balto HA. Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites. 2022 Sep 27;12(10):912. doi: 10.3390/metabo12100912. PMID: 36295814; PMCID: PMC9611210.
- Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Further Evidence for the Use of Aspirin in COVID-19. Int. J. Cardiol. 2022, 346, 107–108.
- Tran, H.T.T.; Gigl, M.; Le, N.P.K.; Dawid, C.; Lamy, E. In Vitro Effect of Taraxacum Officinale Leaf Aqueous Extract on the Interaction between ACE2 Cell Surface Receptor and SARS-CoV-2 Spike Protein D614 and Four Mutants. Pharmaceuticals 2021, 14,1055.
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-ard, P. A Comprehensive Review of Andrographis Paniculata (Burm. F.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules 2022, 27, 4479.
- Sa-Ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J.; et al. Anti-SARS-CoV-2 Activity of Andrographis Paniculata Extract and Its Major Component Andrographolide in Human Lung Epithelial Cells and Cytotoxicity Evaluation in Major Organ Cell Representatives. J. Nat. Prod. 2021, 84, 1261–1270.
- Murugan, N.A.; Pandian, C.J.; Jeyakanthan, J. Computational Investigation on Andrographis Paniculata Phytochemicals to Evaluate Their Potency against SARS-CoV-2 in Comparison to Known Antiviral Compounds in Drug Trials. J. Biomol. Struct. Dyn. 2021, 39, 4415–4426.
- Halma MTJ, Plothe C, Marik P, Lawrie TA. Strategies for the Management of Spike Protein-Related Pathology. Microorganisms. 2023 May 17;11(5):1308. doi: 10.3390/microorganisms11051308. PMID: 37317282; PMCID: PMC10222799.