Adjunctive Zinc for Major Depression : A Systematic Review of RCTs
Student Scholarship Honorable Mention
Georgi Stoychev, BSc
Baljit Khamba, ND, Mph
Mental disorders are the leading cause of non-fatal disease burden, globally and nationally. It is estimated that 1 in 4 adults in the United States suffers from anxiety and depression disorders.1 Major depressive disorder (MDD) is the most common type of depression, affecting 6.7% of the population in a given year.2
Depression is related to chronic disease. Clinical obesity raises the risk of developing MDD by 55%.3 Individuals who are depressed are more likely to subsequently develop obesity, type 2 diabetes, and coronary heart disease.3-5 MDD is also independently associated with poor treatment adherence, which is crucial to the success of naturopathic recommendations.6-8
The Diagnostic and Statistical Manual of Mental Disorders (DSM) sets diagnostic criteria for mental disorders in the United States. MDD is characterized by the following: (1) persistent low mood and (2) loss of interest or pleasure, and accompanied by somatic symptoms such as significant changes in (3) weight, (4) appetite, (5) sleep, and (6) cognition.9
The monoamine deficiency hypothesis is a widely accepted explanation for the pathophysiology of depression. Low levels of serotonin, norepinephrine, and dopamine are characteristic of depression, and replenishment with antidepressants improves symptoms in severe MDD.10,11 However, minimal benefit is reported in mild-to-moderate depression.12 This is further complicated by low response and remission rates and high recurrence (“treatment-resistance”).13-15
Rationale for Using Zinc in MDD
Zinc saturates the hippocampus and the grey matter of the cerebral cortex.16 In MDD, moderate-to-severe shrinkage of these areas is observed on imaging studies.17,18 On a molecular level, zinc is a partial N-methyl-D-aspartate (NMDA) receptor antagonist, thus preventing the excessive neuron stimulation that may be present in MDD.19 Individuals with depression have lower plasma zinc levels compared to healthy controls.20 An earlier systematic review found adjunctive zinc administration with antidepressants to be beneficial in improving depressive symptoms; however, conclusions were limited by inadequately powered randomized controlled trials (RCTs).21
Literature Review
In order to assess the effects of zinc compared to placebo as an add-on therapy to antidepressants for treating MDD in adults, we conducted a systematic-type review of existing research. Table 1 lists our criteria for selecting studies in our review. Consistent with an evidence-based medicine approach, we utilized a PICO approach in our analysis (P=population; I=intervention; C=control; O=outcome).
Table 1. PICO Analysis & Eligibility Criteria for Review
| Category | Inclusion/Exclusion Criteria |
| Study type | RCTs with human subjects, published and unpublished ≥1 week
English language studies |
| P
(Population) |
Men and women ≥18 years
MDD diagnosis, with or without psychosis and other comorbidities, according to DSM-IV/V or International Statistical Classification of Diseases and Related Health problems, 10th revision (ICD-10) |
| I
(Intervention)
|
Zinc as add-on therapy to antidepressants
Excluded zinc as monotherapy or as part of multivitamin or mineral complex |
| C
(Control) |
Placebo as control |
| O
(Outcome) |
Primary outcomes:
1. Changes in mean score on depression scales, from baseline to end 2. Participant drop-out rates Secondary outcomes: 3. Response (50% or greater reduction in depression score at study endpoint) 4. Remission (depression score within normal range at study conclusion) 5. Number and nature of adverse effects |
Search Methods
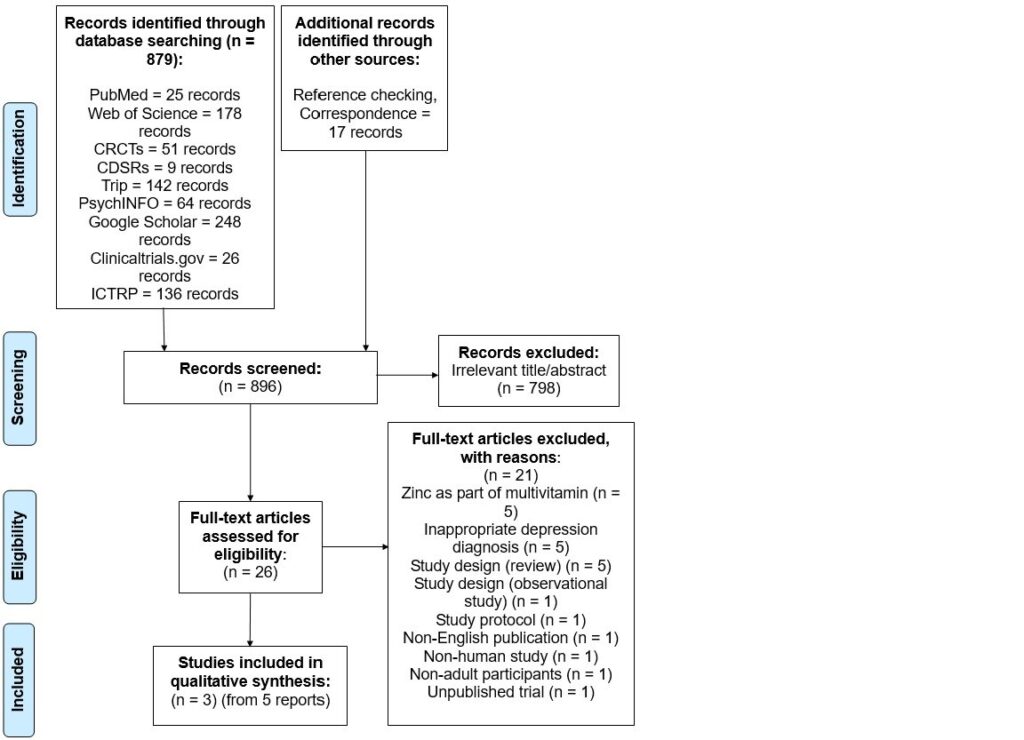
Seven databases were searched, from all years of record until January 2017 (Figure 1). The databases included the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, PubMed, Web of Science, Google Scholar, PsychINFO, and Trip. International trial registries, including ClinicalTrials.gov and WHO International Clinical Trials Registry Platform, were also searched for unpublished or ongoing trials. Reference lists were checked and experts in the field were contacted.
Figure 1. PRISMA Diagram of Search & Study Selection Process
Data Collection & Analysis
One author independently conducted data extraction, risk of bias, and quality-of-evidence assessment. This was done through the Cochrane Collaboration’s data extraction form, risk of bias tool, and the GRADE framework.22,23 Studies were judged as high, low, or unclear risk of bias (Figures 2,3). Evidence for each outcome was graded as high, medium, low, or very low quality.
Figure 2. Risk of Bias; Percentages
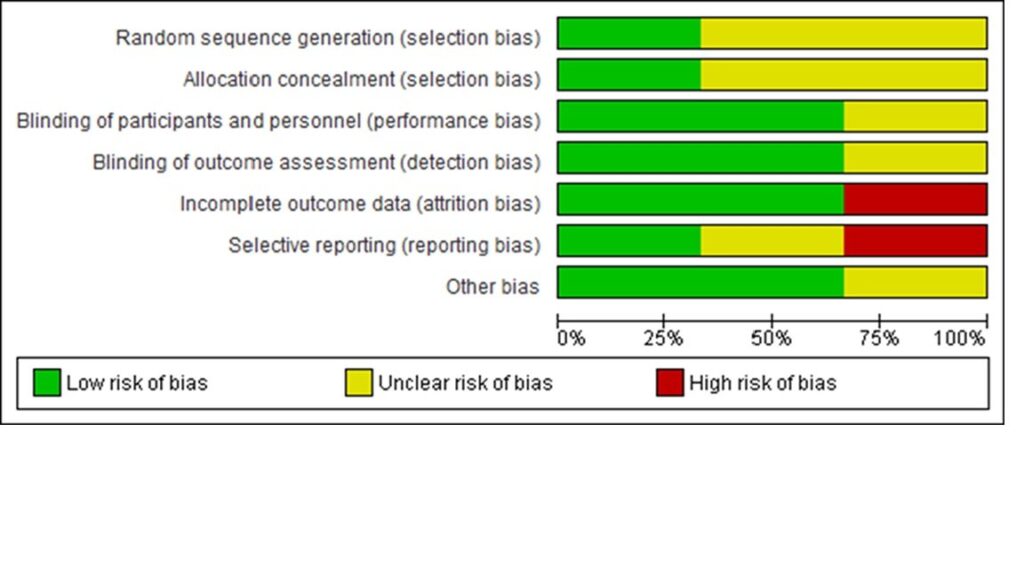
Figure 3. Risk of Bias: Category Judgments
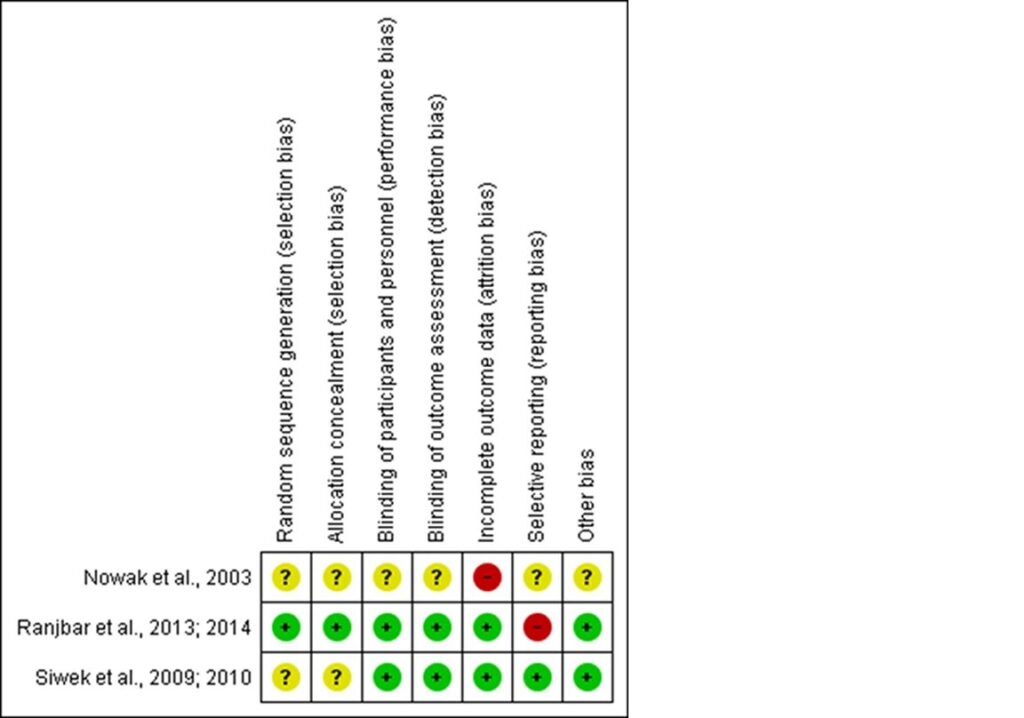
Three studies were selected for review. Most of these studies were determined to be at low risk of performance, detection, and attrition bias, unclear risk of selection bias, and mixed risk of reporting bias. Two trials ensured rigorous measures of minimizing bias overall,24-27 while the third study had poor reporting of methodology and was ascribed unclear risk of bias for most categories.28 The quality of evidence23 for all outcomes ranged from low to very low (see Table 2).
Table 2. Findings & Quality of Evidence
| Outcome | Number of Participants in Studies | Result
(Zinc compared to placebo) |
Quality of Evidence (GRADE) |
| Change in mean scores on depression scales | 103 (3 studies)24-28 | Zinc significantly superior | ⊕⊕⊝⊝
low |
| Participant drop-outs | 103 (3 studies)24-28 | Non-significantly lower in zinc | ⊝⊝⊝⊝
very low |
| Response rate | 52 (1 study)26,27 | No significant difference | ⊕⊝⊝⊝
very low |
| Remission rate | 52 (1 study)26,27 | No significant difference | ⊕⊝⊝⊝
very low |
| Adverse effects | 52 (1 study)26,27 | No significant difference | ⊝⊝⊝⊝
very low |
Discussion
The quality of the evidence for every outcome was rated as follows: risk of bias, indirectness, inconsistency, imprecision, and publication bias. (See Table 3)
Points were downgraded for risk of bias (high for 2 categories), indirectness (only 2 countries represented), and imprecision (small sample size and number of events).
Table 3. Completeness & Applicability of Findings
| Category | Comments |
| P | Studies conducted in Poland and Iran only
Depression subgroups not considered Treatment-resistant subgroup explored in only 1 study Male-to-female ratio in studies matched depression gender distribution in general population No ICD-10-diagnosed depression; however, high concordance between DSM-5 and ICD-1029 |
| I
|
Only zinc hydroaspartate and sulphate examined
Limited classes of antidepressants used |
| C | Compared to placebo in all studies |
| O | Insufficient information on clinically relevant outcomes, eg, response and remission rates, adverse effects |
Biases in Review Process
- Strengths: Detailed search strategy; use of “gold-standard” methodology for data extraction, risk of bias, and quality-of-evidence assessment; multiple outcome measures; Cochrane Collaboration Group structure
- Limitations: No grey literature search; single study author (limits search, data extraction, risk of bias, and quality-of-evidence assessment); no meta-analysis; however, mini meta-analysis informed quality-of-evidence assessment
Agreement with Other Reviews/Studies
- Similarities: Similar PICO, eg, adults with MDD, zinc as add-on therapy, placebo as comparator, reduction in scores on depression scales21
- Differences: 2012 review also examined zinc in healthy adults and as part of multivitamins; this review included 1 new study24,25; more clinically relevant outcomes (Table 2); more rigorous and up-to-date methodology for risk of bias and quality-of-evidence assessment
Conclusion
Given the absence of moderate-to-high-quality evidence, firm conclusions about zinc as an add-on therapy to antidepressants for treating MDD cannot be made at this time. Further evidence from larger, higher-quality RCTs is necessary, with particular focus on adequate sequence generation, allocation concealment, handling of missing data, and avoidance of selective outcome reporting. Trials should encompass a variety of participants, settings, antidepressant subclasses, and patient-centered outcome measures. Healthcare professionals in mental health, including naturopathic doctors, should be mindful of zinc status in patients, given the higher prevalence of zinc deficiency in MDD, and address any imbalances found.
References:
- Anxiety and Depression Association of America. Facts & Statistics. August 2017. Available at: https://adaa.org/about-adaa/press-room/facts-statistics. Accessed September 7, 2017.
- Bose J, Hedden SL, Lipari RN, et al. Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. September 2016. Substance Abuse and Mental Health Services Administration Web site. https://tinyurl.com/y9avanck. Accessed September 7, 2017.
- Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression. Arch Gen Psychiatry. 2010;67(3):220-229.
- Gan Y, Gong Y, Tong X, et al. Depression and risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2014;14:371.
- Yu M, Zhang X, Lu F, et al. Depression and risk for diabetes: A Meta-Analysis. Can J Diabetes. 2015;39(4):266-272.
- Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398-2403.
- Somerset SM, Graham L, Markwell K. Depression scores predict adherence in a dietary weight loss intervention trial. Clin Nutr. 2011;30(5):593-598.
- Trief PM, Cibula D, Delahanty LM, Weinstock RS. Depression, stress, and weight loss in individuals with metabolic syndrome in SHINE, a DPP translation study. Obesity. 2014;22(12):2532-2538.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
- Delgado P. Depression: The Case for a Monoamine Deficiency. J Clin Psychiatry. 2000;61(6):7-11.
- Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity. JAMA. 2010;303(1):47-53.
- Kirsch I, Deacon BJ, Huedo-Medina TB, et al. Initial severity and antidepressant benefits: A Meta-Analysis of data submitted to the food and drug administration. PLoS Medicine. 2008;5(2):e45.
- Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
- Nemeroff C. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007;68(8):17-25.
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
- Hu KH, Friede RL. Topographic determination of zinc in human brain by atomic absorption spectrophotometry. J Neurochem. 1968;15(7):677-685.
- Koolschijn P, van Haren N, Lensvelt-Mulders G, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30(11):3719-3735.
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957-1966.
- Petrilli MA, Kranz TM, Kleinhaus K, et al. The Emerging Role for Zinc in Depression and Psychosis. Front Pharmacol. 2017;8:414.
- Swardfager W, Herrmann N, Mazereeuw G, et al. Zinc in depression: a meta-analysis. Biol Psychiatry. 2013;74(12):872-878.
- Lai J, Moxey A, Nowak G, et al. The efficacy of zinc supplementation in depression: systematic review of randomised controlled trials. J Affect Disord. 2012;136(1-2):31-39.
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0. Updated March 2011. The Cochrane Collaboration; 2011. Available from: http://training.cochrane.org/handbook. Accessed September 7, 2017.
- Schünemann H, Brożek J, Guyatt G, Oxman A, eds. GRADE Handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group; 2013. Available at: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed September 7, 2017.
- Ranjbar E, Kasaei MS, Mohammad-Shirazi M, et al. Effects of zinc supplementation in patients with major depression: a randomixed clinical trial. Iran J Psychiatry. 2013;8(2):73-79.
- Ranjbar E, Shams J, Sabetkasaei M, et al. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr Neurosci. 2014;17(2):65-71.
- Siwek M, Dudek D, Paul IA, et al. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord. 2009;118(1-3):187-195.
- Siwek M, Dudek D, Schlegel-Zawadzka M, et al. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J Affect Disord. 2010;126(3):447-452.
- Nowak G, Siwek M, Dudek D, et al. Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol J Pharmacol. 2003;55(6):1143-1147.
- Andrews G, Slade T, Peters L. Classification in psychiatry: ICD-10 versus DSM-IV. Br J of Psychiatry. 1999;174(1):3-5.
Acknowledgements:
Thanks to Dr Dimple Radia, lecturer at London Metropolitan University, for her extensive support and supervision while conducting this undergraduate research project.
 Georgi Stoychev, BSc, is a dietetics graduate from London, UK; he obtained his BSc at London Metropolitan University. While working toward his dietetics degree, he was able to see a variety of patients with nutrition-related disorders. Presently, Georgi is a student of naturopathic medicine at Bastyr University California. He has a particular interest in naturopathic approaches to mental health. As part of broadening our understanding in this area, he stays abreast of research on naturopathic treatment options for mental health conditions.
Georgi Stoychev, BSc, is a dietetics graduate from London, UK; he obtained his BSc at London Metropolitan University. While working toward his dietetics degree, he was able to see a variety of patients with nutrition-related disorders. Presently, Georgi is a student of naturopathic medicine at Bastyr University California. He has a particular interest in naturopathic approaches to mental health. As part of broadening our understanding in this area, he stays abreast of research on naturopathic treatment options for mental health conditions.
***
 Baljit Khamba, ND, MPH, is a licensed naturopathic doctor in California. Dr Khamba completed her (honors) Bachelor of Science degree (specializing in psychology), as well as her Masters in Public Health degree, at York University, in Toronto, Canada. She received her naturopathic doctoral degree from the Canadian College of Naturopathic Medicine (CCNM), also in Toronto. She was also involved with research projects at the University of Alberta on natural health product safety. Dr Khamba is a member of the American Osteopathic Association of Prolotherapy Regenerative Medicine.
Baljit Khamba, ND, MPH, is a licensed naturopathic doctor in California. Dr Khamba completed her (honors) Bachelor of Science degree (specializing in psychology), as well as her Masters in Public Health degree, at York University, in Toronto, Canada. She received her naturopathic doctoral degree from the Canadian College of Naturopathic Medicine (CCNM), also in Toronto. She was also involved with research projects at the University of Alberta on natural health product safety. Dr Khamba is a member of the American Osteopathic Association of Prolotherapy Regenerative Medicine.