Author: Robert Sheaff, PhD, and Ian Mitchell
Abstract
This study investigated whether quantum field exposure generated by Quantum Upgrade based on Leela Quantum technology, influences adenosine triphosphate (ATP) production in human cell lines. This double-blind experiment was performed using A549 human lung carcinoma cells and immortalized human diploid fibroblasts (HDFs). ATP was quantified using the CellTiter-Glo assay following remote quantum field treatment applied from over 500 miles away. Treated cells exhibited transient but statistically significant elevations in ATP production, ranging from 20 to 29 percent. The findings suggest that non-local quantum field exposure may modulate cellular bioenergetics. Additional replication and mechanistic work are required to confirm these observations and elucidate underlying pathways.
Introduction
Adenosine triphosphate (ATP) serves as the universal energy currency of the cell, driving nearly all anabolic reactions, signal transduction pathways, and mechanical work within living systems. Because ATP is continually utilized and replenished, its steady-state levels provide a sensitive indicator of the cell’s bioenergetic balance.
Leela Quantum Tech has developed a proprietary technology designed to project quantum energy fields capable of interacting with biological systems. Quantum Upgrade functions through subtle informational fields rather than electromagnetic or mechanical signals. These fields carry highly coherent energetic patterns that interact with the body’s intrinsic quantum communication systems. This interaction appears to help restore biological coherence and optimize cellular and systemic regulation. The system works by providing a stable coherence field that the body can resonate with to support balance and overall well-being.
Materials and Methods
Two human cell lines were used: A549 lung carcinoma cells and human diploid fibroblasts (HDFs). Cells were cultured in DMEM with standard supplements at 37 °C in a humidified incubator with 5% CO₂.
An independent, double-blind experiment was conducted. Two groups of cells—randomly labeled “A” and “B”—were prepared identically; one was exposed to the quantum field remotely (>500 miles away), while the other served as the control. Neither the experimenter nor staff knew which group was treated until analysis completion.
Measurements were conducted in quadruplicate, and statistical analysis was performed using ANOVA with p < 0.05 considered significant.
Results
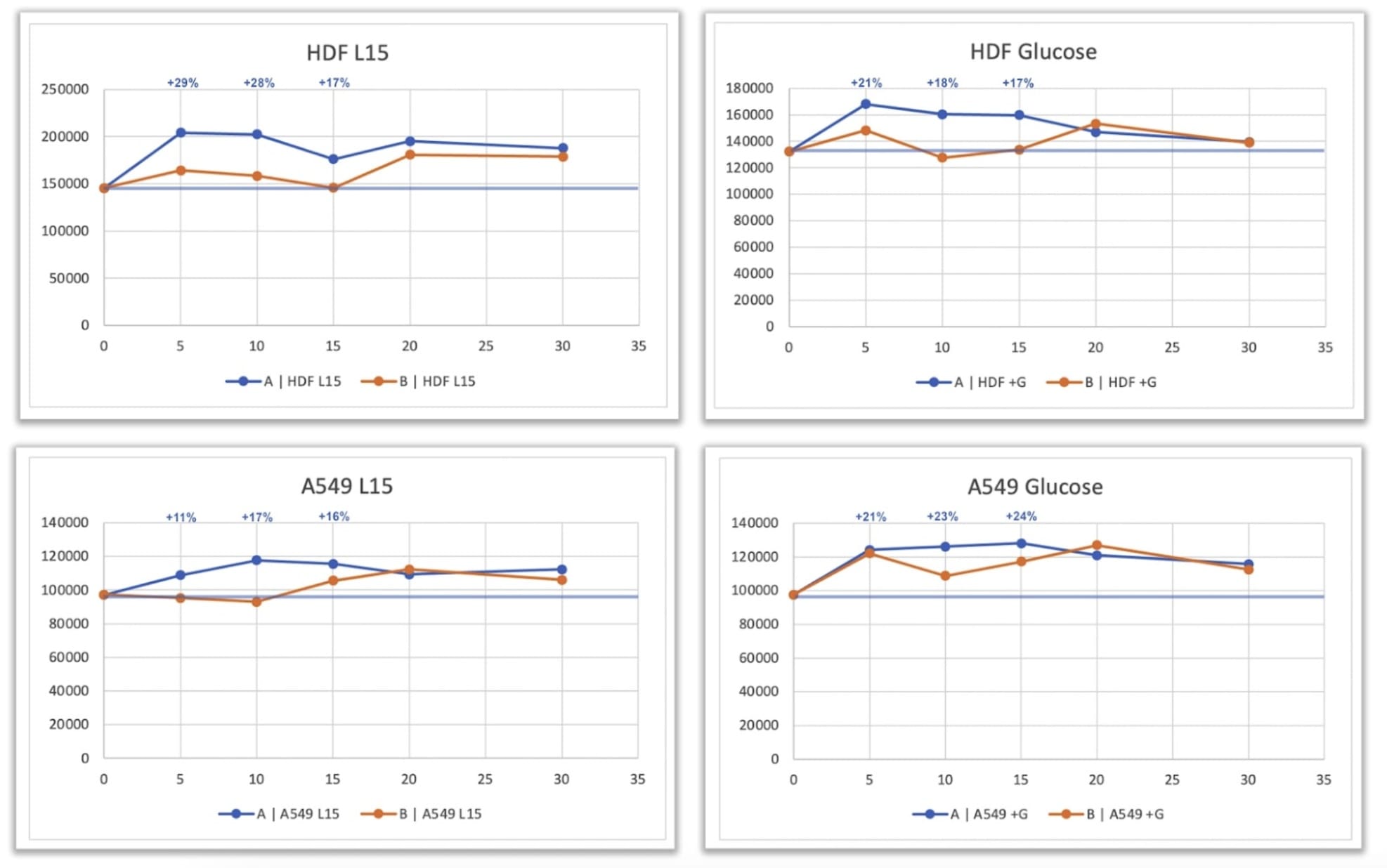
A549 and HDF cells were distributed into two separate 96-well plates specifically designed to allow cell attachment. The use of attachment plates ensured proper adherence of both cell types prior to analysis. To prevent fluctuations in pH that could occur with repeated removal of plates from a CO₂ incubator, the experiment was performed at 37 °C in the absence of CO₂. For these conditions, L15 medium with or without glucose was used, as it is formulated for culture under non-CO₂ conditions.
Following plating, cells were incubated for seven hours at 37 °C to allow attachment, confirmed by microscopy, but not long enough for cell replication. After incubation, the plates were randomly labeled “A” and “B” and transferred to a colleague who performed the quantum exposure procedure in collaboration with Quantum Upgrade based on Leela Quantum Tech personnel located more than 500 miles away. One plate was exposed to the quantum field (“treated”), and the other served as the control (“untreated”). This stage of the experiment was performed entirely in the absence of the principal investigator to preserve a double-blind design. Within five minutes, both plates were returned to the incubator, and at predefined time points, ATP levels were analyzed using the CellTiter-Glo® (CTG) luminescent assay applied directly to each well. Measurements were performed in quadruplicate at each time point to improve data quality and statistical reliability.
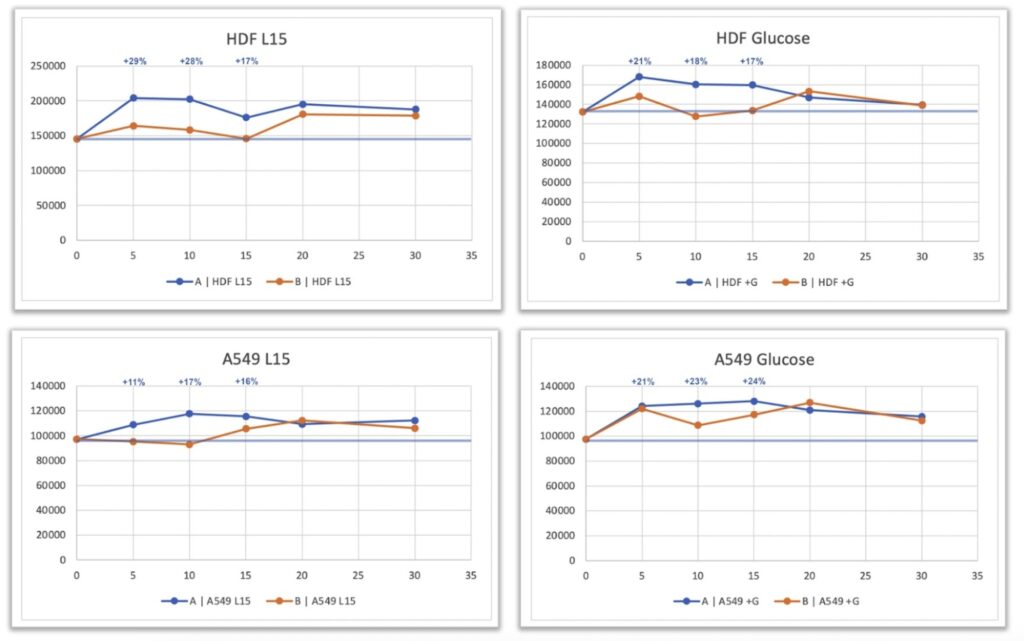
A transient but statistically significant increase in ATP levels was observed in the treated cells compared with the untreated controls. This increase occurred in both the presence and absence of glucose, indicating that the observed effect was not restricted to a specific metabolic pathway. In A549 cells, ATP levels increased by approximately 20 – 29 %, while HDF cells also showed a transient elevation in ATP concentration, particularly in the glucose-containing condition.
Although the signal in HDFs was less pronounced than in A549 cells, the trend toward higher ATP production in treated samples was clearly evident. The removal of the “spill-over” effect—previously identified as possible cross-influence between charged and uncharged plates—likely contributed to the stronger response observed in this optimized configuration, where untreated cells remained entirely unexposed to the field.
Discussion
The results of this experiment indicate that exposure of human cells to a remotely applied quantum field generated by Quantum Upgrade based on Leela Quantum Tech technology was associated with a measurable, transient increase in ATP production. Both A549 and HDF cell lines demonstrated this response under carefully controlled, double-blind conditions, suggesting that the phenomenon is reproducible across metabolically distinct cell types.
The increase in ATP levels observed in treated cells, ranging from approximately 20 to 29 percent, reflects a biologically meaningful modulation of cellular energy metabolism. The fact that similar changes were detected under both glucose-containing and glucose-free conditions suggests that the effect is not dependent on a specific metabolic pathway. Instead, it may represent a generalized enhancement of cellular energy production or efficiency, potentially involving mitochondrial or enzymatic regulation.
The experiment was designed to minimize confounding factors that could influence ATP measurements. Conducting the study in a non-CO₂ incubator prevented fluctuations in pH caused by repeated removal of plates, which are known to affect cellular metabolic activity. The use of attachment-compatible plates also ensured consistent cell adherence, a critical factor for fibroblast metabolism and reproducible ATP quantification.
The findings suggest that remote quantum field exposure can transiently modulate cellular bioenergetics. Possible explanations could involve modulation of mitochondrial function, transient shifts in membrane potential, or resonance effects influencing enzymatic activity within the oxidative phosphorylation system. These possibilities remain speculative and require targeted investigation using direct mitochondrial and molecular assays.
The double-blind structure of the study, together with consistent replication across two human cell lines, supports the reliability of the observed response. Future work should address these gaps by including larger sample sizes, detailed statistical validation, and independent replication in additional laboratories.
Conclusion
Under rigorously controlled, double-blind laboratory conditions, exposure of human A549 and HDF cells to a remotely generated quantum field produced by Quantum Upgrade based on Leela Quantum Tech technology was associated with a transient increase in ATP levels. The response was observed in both glucose-containing and glucose-free environments, indicating that the effect was not limited to a specific metabolic pathway. The magnitude of change—approximately 20–29 percent above control levels—suggests a measurable modulation of cellular bioenergetics.
While the exact mechanism remains undetermined, the findings provide preliminary evidence that quantum field exposure may transiently influence energy production in living cells. These results warrant further investigation using expanded sample sizes, detailed statistical evaluation, and independent replication to confirm reproducibility and explore underlying biophysical pathways.

Robert Sheaff, PhD, Associate professor of biochemistry at The University of Tulsa. Received a B.A. in biology and philosophy from the University of North Carolina at Chapel Hill in 1989, a Ph.D. in chemistry from the University of Colorado at Boulder in 1994, and did postdoctoral work at the Fred Hutchinson Cancer Research Center in Seattle. Current research interests are role of the tumor suppressor p27kip1 and drug discovery/characterization.

Ian Mitchell, CSO, Wizard Sciences, biochemist, and pharmaceutical developer who specializes in anti-aging technology and peak performance.
References
- Nicholls, D. G., & Ferguson, S. J. (2013). Bioenergetics 4. Academic Press.
- Berg, J. M., Tymoczko, J. L., & Stryer, L. (2019). Biochemistry (9th ed.). W. H. Freeman.
- Brand, M. D., & Nicholls, D. G. (2011). Assessing mitochondrial dysfunction in cells. Biochemical Journal, 435(2), 297–312.
- Wallace, D. C. (2012). Mitochondria and cancer. Nature Reviews Cancer, 12(10), 685–698.
- Balaban, R. S., Nemoto, S., & Finkel, T. (2005). Mitochondria, oxidants, and aging. Cell, 120(4), 483–495.
- Goodman, R., & Blank, M. (2002). Insights into electromagnetic field interactions with living systems. Journal of Cellular Physiology, 192(1), 16–22.
- Schrödinger, E. (1935). Discussion of probability relations between separated systems. Proceedings of the Cambridge Philosophical Society, 31, 555–563.
- Einstein, A., Podolsky, B., & Rosen, N. (1935). Can quantum-mechanical description of physical reality be considered complete? Physical Review, 47(10), 777–780.
- Tegmark, M. (2000). Importance of quantum decoherence in brain processes. Physical Review E, 61(4), 4194–4206.