Immune Function & Competence: Gut Microbial Influences
MICHELLE MADDUX, ND
The intricate interplay between our commensal bacteria and our immune system is complex and wide-reaching. Given that immune competence is at the forefront of our public health interest as of late, this article will briefly review the relationship, mechanisms, and potential therapeutic value of intestinal microbiome manipulation through therapeutic probiotic supplementation.
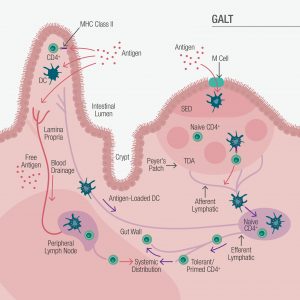
Comprised of the lamina propria, mesenteric lymph nodes, intraepithelial lymphocytes, and lymphoid follicles including Peyer’s patches, the gut-associated lymphoid tissue (GALT) represents 70% of the human immune system (Figure 1).1
Figure 1. Gut-Associated Lymphoid System
In The Beginning
Mother’s influence on fetal microbiome and immune development begins early, with a shift in maternal T-helper cells toward Th2 in support of immune tolerance for her developing baby. At the same time, the neonatal immune system is preparing for microbiota colonization by adopting a regulatory immune environment and blunted inflammatory response.2 Although it was once believed that the uterine environment was sterile, evidence now suggests that mother shares not only growth factors, antibodies, and microbial metabolites transplacentally, but also microbes themselves.3
The developing fetal gut microbiota is influenced by maternal diet, genetics, use of antibiotics, and other factors. However, it is well established that seeding of the infant microbiome is primarily determined by mode of birth. Babies born vaginally are more likely to be colonized by Lactobacillus spp, whereas C-section-delivered children are initially colonized by mother’s skin and oral microbes.4 In addition to setting the stage for the microbial population into adulthood, the infant immune system must shift away from a tolerant Th2 environment to one that features Th1 dominance. The colonizing microbes then communicate with the developing infant immune system through microbial-associated molecular patterns (MAMPs) that help to direct healthy gut microbial balance and immune homeostasis.5 Without this balancing act between gut and immune response, infants are more likely to develop atopy, allergies, and other consequences of immune dysregulation.2,6
Microbe Mechanisms in Immune Competence
Commensal microbes in the gut play a vital role in the training and optimal function of the immune system through a variety of mechanisms. Although a comprehensive discussion of these mechanisms is beyond the scope of this article, several of them are briefly described below.
Colonization Resistance
One of the foundational defensive tools of gut commensals is the resistance of pathogenic colonization.5 This is accomplished via nutrient competition, production of toxic bacteriocins and proteases, and manipulation of pH (as in the case of lactic acid bacteria in the vagina).
Intestinal Barrier Integrity
Because the integrity of the gut epithelial barrier plays such an vital role in both systemic and intestinal health,6 it has been referred to as the “mucosal firewall.”5 Here are some of the key components involved:
- The Mucus Layer5,7: Generated by goblet cells, the intestinal mucus layer, along with the antimicrobial action of intestinal cell defensins, is the primary barrier protecting against translocation of gut microbes. The well-documented antimicrobial lectin, RegIIIγ, is directly controlled by commensal bacteria and targets potentially pathogenic gram-positive organisms. Hyperglycosylated mucin 2 (MUC2), the basis of this protective layer, also plays a role in dendritic-cell antigen presentation.
- Antigen Presentation – Antibody Production5,6: Another tool in the arsenal is IgA, which is produced with the assistance of enteric dendritic cells that sample and present gut organisms to Peyer’s patches B and T cells. Intestinal secretory IgA, along with antimicrobial peptides (AMPs), are integral to effective barrier function.
- Short-Chain Fatty Acid Production5,7: Byproducts of microbial carbohydrate metabolism, short-chain fatty acids (SCFAs) mainly consist of acetate, propionate, and butyrate, the last of which provides major enterocyte nutrition, thereby supporting intestinal barrier integrity. Butyrate also plays a direct role in regulatory T-cell (Treg) induction. SCFAs further combat invasive organisms by altering intracellular pH and interfering with microbial metabolism.
Zonulin Production
A peptide strongly associated with tight junction integrity, increased zonulin levels are associated with dysbiosis, inflammation, and inflammatory disease states.8,9 In a vicious cycle, gut dysbiosis leads to further zonulin release and proinflammatory cytokine production that continue to erode barrier function.9
Regulatory Cell Induction
The microbiota plays a complicated and dynamic role in shaping immune response through the induction of regulatory and effector T-cells, including Tregs and Th17.5,8
Cytokine Production
Commensal bacteria play a role in innate immune defense by influencing the production of various cytokines, including the production of interleukin (IL)-1β, critical to host defense response.5 Gut microbes are also involved in the production and regulation of various pro- and anti-inflammatory cytokines, including tumor necrosis factor (TNF)-α, IL-6, IL-10, and others.10,11
Immune Tolerance
Gut microbes are integral to establishing tolerance to ingested and environmental antigens for optimal homeostasis. Gastrointestinal-induced Foxp3 Treg cells, in particular, are a key component of maintaining balanced tolerance.5
The Microbiome in Immune-Mediated Disease
Despite, or perhaps because of, the multitude of microbial immune-supportive mechanisms, microbiome imbalance (dysbiosis) has been associated with a wide variety of conditions, ranging from antibiotic-associated diarrhea to immune-mediated disease. The following are common examples:
Antibiotic-Associated Diarrhea
Antibiotics, especially those targeted toward anaerobes – including aminopenicillins, cephalosporins, and clindamycin – result in antibiotic-associated diarrhea (AAD) in as many as 39% of cases.12 In a 2017 review, 17 randomized controlled trials were examined to evaluate probiotic efficacy in treating AAD.12 Despite inconsistencies in study design, duration, and intervention type, the findings suggested that probiotic use reduces AAD risk by more than 50%.
Atopic Dermatitis & Allergy
The frequency of atopic dermatitis (AD) has been on the rise in recent decades and is often seen as the beginning of the “allergic march,” leading to asthma in half of cases and allergic rhinitis in two-thirds.13 Decreased Bifidobacterium spp and IL-10 production, along with increased proinflammatory cytokine production, is seen with children suffering with this condition.14 Although an abundance of research has been conducted to evaluate the efficacy of probiotics in AD, study design, intervention formula, and results have varied.15 Of interest, one longitudinal study, evaluating a 3-strain probiotic formulation, demonstrated increased IL-10 and IFN-γ, reduced IL-4, IL-5, IL-13, and a 58% relative risk reduction in high-risk children over a 2-year period.16
Inflammatory Bowel Disease
Although not fully elucidated, dysbiosis leading to hyperpermeability and a hyperinflammatory state has been associated with the development of inflammatory bowel disease (IBD).17 Probiotic manipulation of the microbiome in IBD has yielded some positive results, including a 2020 meta-analytical review of 19 studies (1537 patients), which found consistent, significant improvement from probiotics in ulcerative colitis alone.18
Autoimmunity/Type 1 Diabetes
Dysbiosis is found in some autoimmune diseases, which is thought to contribute to impaired epithelial barrier function, exaggerated inflammatory response, and decreased Treg cells.19 Because commensals have such a significant influence on immune function, the adjuvant effect of probiotics has been evaluated in islet autoimmunity (IA). In the 2016 prospective cohort TEDDY study (7473 children), investigators found that early probiotic supplementation decreases the risk of type 1 diabetes in children at high genetic risk.20
In the End
As we age, Bifidobacterium and Clostridiales decrease, while Proteobacteria and Enterobacteriaceae increase, leading to age-related dysbiosis (“microb-aging”) and increased inflammatory response (“inflammaging”).21 These changes are among the hypothesized drivers of immunosenescence and increased risk of infection with aging. A 2021 article discusses these phenomena and suggests that probiotic formulations, including strains demonstrated to influence immunity and inflammatory response (see sidebar) can be effective tools to defend epithelial barrier strength, microbial diversity, and healthier aging overall.21
Selecting an Immune-Supporting Probiotic
Not all probiotic strains perform in the same way, and not all commercial formulations support reliable metabolic activity and synergistic mechanisms. Although the immune-mediating effects of select strains make probiotics a compelling clinical tool, identification of an effective formulation can be challenging. It is therefore incumbent upon manufacturers to provide practitioners both in-vitro strain characterization and in-vivo human clinical data on the final formulation. Requesting this information will raise industry quality standards, provide evidence of a properly designed product, as well as offer greater confidence in the likelihood of achieving the desired outcomes.
References
- Vighi G, Marcucci F, Sensi L, et al. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153 Suppl 1(Suppl 1):3-6.
- Dzidic M, Boix-Amorós A, Selma-Royo M, et al. Gut Microbiota and Mucosal Immunity in the Neonate. Med Sci (Basel). 2018;6(3):56.
- Vandenplas Y, Carnielli VP, Ksiazyk J, et al. Factors affecting early-life intestinal microbiota development. Nutrition. 2020;78:110812.
- Dunn AB, Jordan S, Baker BJ, Carlson NS. The Maternal Infant Microbiome: Considerations for Labor and Birth. MCN Am J Matern Child Nurs. 2017;42(6):318-325.
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121-141.
- Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492-506.
- Ducarmon QR, Zwittink RD, Hornung BVH, et al. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol Mol Biol Rev. 2019;83(3): e00007-19.
- Tajik N, Frech M, Schulz O, et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat Commun. 2020;11(1):1995.
- Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:F1000 Faculty Rev-69.
- Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17(20):7618.
- Alexander KL, Targan SR, Elson CO 3rd. Microbiota activation and regulation of innate and adaptive immunity. Immunol Rev. 2014;260(1):206-220.
- Blaabjerg S, Artzi DM, Aabenhus R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients-A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2017;6(4):21.
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118-S127.
- He F, Morita H, Hashimoto H, et al. Intestinal Bifidobacterium species induce varying cytokine production. J Allergy Clin Immunol. 2002;109(6):1035-1036.
- Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. Int Forum Allergy Rhinol. 2015;5(6):524-532.
- Niers L, Martín R, Rijkers G, et al. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy. 2009;64(9):1349-1358.
- Lee M, Chang EB. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology. 2021;160(2):524-537.
- Pabón-Carrasco M, Ramirez-Baena L, Vilar-Palomo S, et al. Probiotics as a Coadjuvant Factor in Active or Quiescent Inflammatory Bowel Disease of Adults-A Meta-Analytical Study. Nutrients. 2020;12(9):2628.
- Xu H, Liu M, Cao J, et al. The Dynamic Interplay between the Gut Microbiota and Autoimmune Diseases. J Immunol Res. 2019;2019:7546047.
- Uusitalo U, Liu X, Yang J, et al. TEDDY Study Group. Association of Early Exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr. 2016;170(1):20-28.
- Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021 Apr 19:1-15. doi: 10.1038/s41435-021-00126-8. [Epub ahead of print]

Michelle Maddux, ND received her doctorate of naturopathic medicine from Southwest College of Naturopathic Medicine (SCNM). Dr Maddux has completed additional training in integrative and functional medicine, laboratory medicine, eating disorders, meditation, yoga, and mindfulness. She is the founder and CEO of Soaring Phoenix, a consulting firm dedicated to educating on the fundamentals of integrative and functional medicine. Dr Maddux is passionate about changing the paradigm of medicine and believes education is the key to making that happen.