Tolle Totum
Eric Yarnell, ND, RH (AHG)
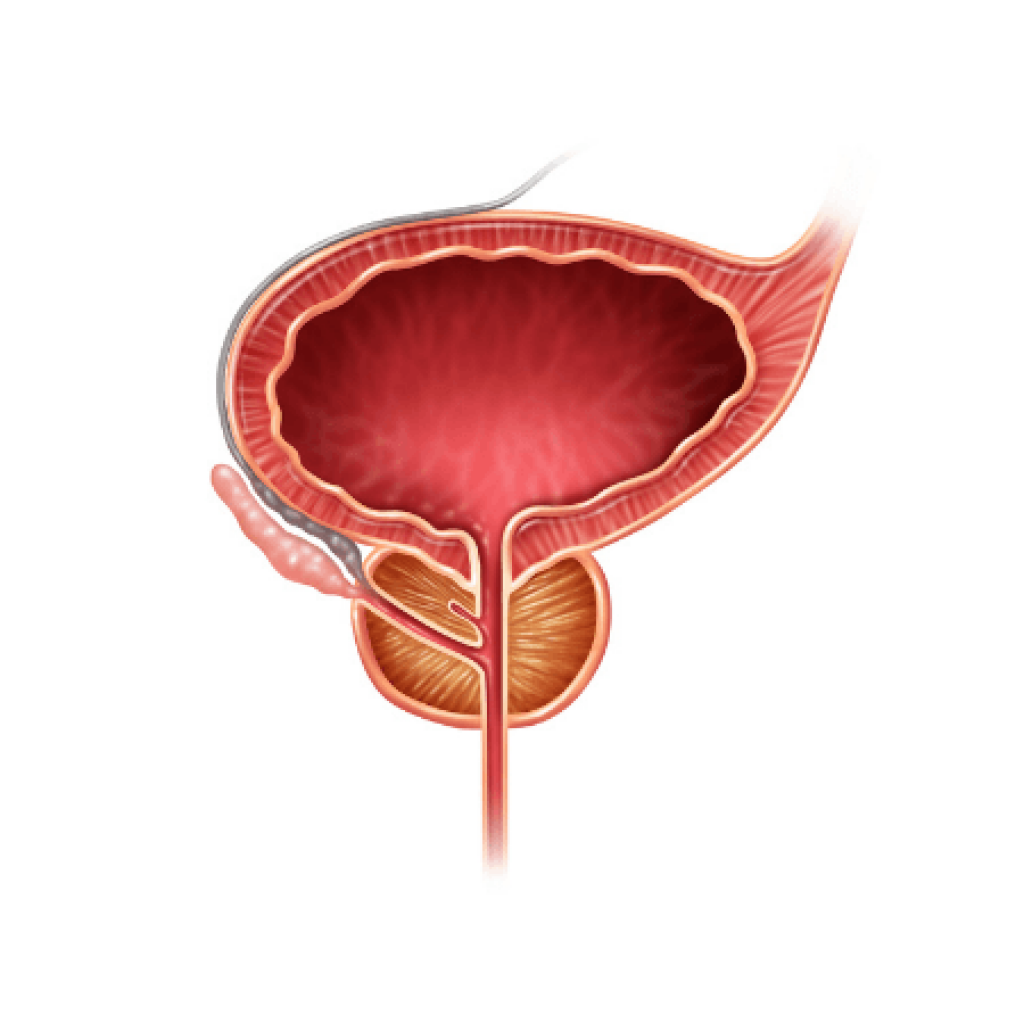
The common condition benign prostatic hyperplasia (BPH) remains relatively poorly understood clinically. There is much room for improvement, including a long way to go beyond just providing Serenoa repens (saw palmetto) and clearing up all the misunderstanding of this herb’s role, in general. In this article, the causes of BPH will be discussed along with approaches to preventing the problem. Clinical phenotypes of BPH, once it does occur, will be discussed in detail, and the appropriate treatment for patients of different types will be presented.
Preventing BPH
BPH is a complex process that occurs inevitably in all people with prostates as they age, based on data obtained from men all over the world.1 Thus, the prostate will inevitably change with age, though the extent to which it becomes clinically relevant is variable. Hyperplasia occurs in any or all of the cells in the prostate. Western lifestyle is strongly correlated with clinically relevant BPH. This is most strongly supported by data from China, in which populations leading a traditional Chinese lifestyle have dramatically less clinical BPH than those leading westernized lifestyles.2 No single factor in the western lifestyle has definitively been proven to be crucial; rather, it appears to be some constellation of high intake of animal products, refined carbohydrates, and calories coupled with low intake of vegetables, legumes, nuts and seeds, insufficient sleep, sedentariness, unrelenting psychoemotional stress, overwork, and pollution.
Therefore, preventing BPH arguably entails the same approach as that for preventing atherosclerosis and its complications and most common western cancers. Even modest increases in exercise (2-3 hours per week of walking) can substantially reduce the risk of developing clinically relevant BPH.3 Higher vegetable intake appears protective against symptomatic BPH.4 In one small, open clinical trial, switching to a high-fiber/low-animal-product diet along with regular exercise led to reductions in prostatic epithelial hyperplasia within just 2 weeks.5
Estrogens and xenoestrogenic chemicals may also drive BPH. The natural, mild lowering of testosterone levels while estrogen levels rise, as a result of age-induced increased adiposity, may contribute to BPH. Higher endogenous estrogen levels correlate with larger prostates and increased risk of surgery for BPH.6,7 It is unknown if this is because of a relative increase in estrogen compared to youth, or absolute estrogen levels. The role of xenoestrogenic chemicals is also suspected given their recent, rapid rise in the environment correlating with increased frequency and severity of BPH. Avoiding xenoestrogens as much as possible, adopting lifestyle measures to minimize adiposity, and regularly eating phytoestrogenic legumes, nuts, seeds, and whole grains are all reasonable measures to prevent BPH. Higher intake of phytoestrogens from the diet has fairly consistently been shown to protect against clinical BPH.8,9
Lifestyle-based prevention ideally needs to start relatively early in life. While it may feel strange to talk to teenage boys and young men about eating well and exercising regularly partly to prevent BPH, this would be the ideal audience and time to initiate these efforts. However, there is some evidence, as noted above, that starting lifestyle changes later in life can still make a significant difference, and thus should be part of every patient’s treatment protocol.
Spasmodic BPH
Some patients with BPH have purely spasmodic or irritative symptoms, meaning urinary frequency, urgency, and nocturia. Often these patients’ primary concern will be sleep disruption from nocturia. It is imperative to assess whether they are urinating small amounts versus fairly large amounts when they get up, as the treatment is completely different for these situations. Urinating small amounts is consistent with BPH, but also consider chronic prostatitis/interstitial cystitis if there is any pelvic pain or associated burning on urination. Voiding large amounts, on the other hand, can be a sign of nocturnal polyuria due to age-related antidiuretic hormone deficiency10 or can be due to drinking large amounts of liquid at night or taking diuretic drugs.
In cases of purely spasmodic BPH, the prostate generally doesn’t feel particularly large on digital exam, though it is always more firm than a younger prostate. All patients should have a post-void residual ultrasound of the bladder (PVR) at the time of diagnosis. These are offered at all urologists’ offices, although naturopathic physicians in some jurisdictions may be able to perform this test too if they use an automated bladder scanner. If the patient is retaining <100 mL of urine, then this would help confirm the diagnosis of spasmodic BPH. [If the amount retained is ≥100 mL, see the discussion of hormonal BPH below.] In this situation, smooth muscle cells have primarily undergone hyperplasia but not enough to cause substantial enlargement of the gland; however, they are still causing spasmodic symptoms. Another strong clue to the spasmodic phenotype is when patients respond to tamsulosin or other alpha-blockers, which are prostate spasmolytic drugs.
Treating spasmodic BPH obviously centers around the use of moderate-to-strong spasmolytic herbs. Clinically I have found Ammi visnaga (khella) fruit tincture to be very effective for this purpose.1 I start with a dose of 1 mL up to 3 times per day; it may only be needed at bedtime for patients who have nocturia exclusively. The dose can be raised as high as 3 mL 3 times daily (increasing the dose by 1 mL per dose every 5-7 days). I have not observed any adverse effects at these doses. Piscidia piscipula (Jamaican dogwood) bark tincture is equipotent to khella clinically; however, its ecological sustainability is questionable and thus should be considered a second-line therapy. Milder spasmolytics, such as Viburnum spp (cramp bark and black haw) and Dioscorea villosa (wild yam), have simply not been effective in my experience. Piper methysticum (kava) and Pedicularis spp (lousewort) are hit or miss; they simply are not as effective as khella. Combining khella with other spasmolytics hasn’t clearly led to superior outcomes. Sometimes Gelsemium sempervirens (yellow jessamine) root tincture (10-30 drops up to 3 times daily) works when khella or Jamaican dogwood doesn’t. In my experience, most patients who respond to alpha-blocker drugs respond as well or better to khella. I overlap them for 1 week, then have the patient stop the alpha-blocker drug. If symptoms worsen, I start a dose escalation of the khella. The alpha-blocker drug can be restarted at any time if need be.
Role of Saw Palmetto & Pygeum
Spasmolytics may dramatically reduce symptoms, but they do not treat the causes of the symptoms. They must be accompanied by lifestyle changes as discussed previously. In addition, Serenoa repens (saw palmetto) fruit is strongly encouraged. In my experience, this product rarely causes noticeable changes in symptoms for patients, in line with the largest placebo-controlled clinical trials.11 However, this same meta-analysis (among others) concluded that saw palmetto extract is as effective as various drugs for BPH at reducing symptoms, which means either that the drugs are also no more effective than placebo or that something is wrong with the trials involving saw palmetto. The single largest trial ever conducted on saw palmetto (n=1098) showed it was just as effective at finasteride at reducing symptoms, yet safer.12
Longer-term studies (>18 months) have been conducted with saw palmetto, and though none have been double-blind, they consistently demonstrated that saw palmetto prevents progression. Most importantly, a trial including 189 men with mild BPH found that progression of symptoms to the point of requiring surgery was cut by 33% in those taking saw palmetto extract compared to no treatment.13 Therefore I recommend all patients with BPH take saw palmetto extract (320 mg once per day with food) essentially indefinitely, as it is an extremely cheap and extremely safe way to reduce the risk of progression.
Note that I do not recommend ever using Prunus africanum (pygeum) bark. This species is seriously threatened; it is listed in Appendix 2 of the Convention on International Trade in Endangered Species and it continues to be harvested unsustainably.14 Pygeum’s chemistry and actions are similar to those of saw palmetto, and there is no evidence suggesting there is any benefit to using pygeum over saw palmetto or combining them. Even if a guaranteed sustainable source of the bark could be documented for any supplier, it is simply madness to ship the bark of a tree across an ocean when a highly sustainable fruit of a plant can be obtained that does the same thing from much closer. I urge all those reading this document in North America to speak to their supplement suppliers and demand that they stop making products with pygeum.
Hormonal & Mixed BPH
Unfortunately, some men have much larger prostates and develop obstructive symptoms, including difficulty starting and stopping the stream, a slow stream, a feeling of incomplete voiding, and double voiding (urinating very soon after having already gone). These symptoms may be accompanied by irritative symptoms. Men with obstructive symptoms generally have palpably larger, firmer prostates, often with no clearly identifiable sulcus anymore.
The presence of obstructive symptoms generally means that the prostate epithelium has undergone hyperplasia that has usually caused the prostate to grow larger than 50 cc in volume. This is a key cut-point, as the largest trial on saw palmetto showed that the herb did not work in men whose prostates were ≥50 cc.12 I call this phenotype “hormonal BPH,” as the prostate epithelium is the most responsive to hormonal disruption (relative estrogen dominance). In most cases it is truly a mixed picture with some obstructive and some irritative symptoms, but the presence of obstructive symptoms always moves the patient into a different category.
The presence of obstructive symptoms is associated with a greater risk of complications. Some patients with these symptoms may be driven to see a doctor after one of the dangerous complications of BPH – most commonly a urinary tract infection (UTI) or acute urinary retention. Unfortunately, I have also now had 3 patients (all lean men in their mid-60s) who developed acute renal failure due to obstructive BPH. In all 3 cases, this development was a complete surprise, as they had only mild obstructive symptoms or, in 1 case, no such symptoms. All 3 had mild irritative symptoms and were in good health. One had a very strange palpable mass in his lower abdomen, which on CT was ultimately proven to be his bladder, filled with 3500 mL of urine! However, he had no pain and absolutely no symptom suggesting his glomerular filtration rate (GFR) was <15 mL/min. These cases also suggest that some men may simply adapt to their symptoms as they develop slowly over time. Luckily, all 3 of these patients recovered with hospitalization, ongoing catheterization, and nephro-regenerative herbs.
These cases, and others where patients have ignored more severe symptoms and ended up with irreversible bladder hypertrophy requiring chronic intermittent catheterization, have led me to require patients with obstructive symptoms to get a post-void residual (PVR) urine test every year, even if the symptoms are mild. It is crucial to educate men with obstructive BPH about how very serious the situation could be or become.
I have yet to discover a natural therapy that can shrink the prostate. I have tried a wide range of western, Chinese, and Ayurvedic herbs, diet and lifestyle changes, acupuncture, and visceral manipulation. While these measures can help reduce the severity of patients’ symptoms, I have never seen a documented reduction in prostate volume that was clinically significant. Therefore, if the prostate is 50-100 cc in size, I recommend patients do a course of finasteride 5 mg 4 times daily, as this will reliably shrink the prostate up to 50%. Because of the 50% maximum shrinkage issue, prostates over 100 cc in size are unlikely to get below 50 cc and thus are likely to cause chronic obstructive symptoms even with treatment, though the drug may reduce their risk of severe BPH complications. While finasteride can have some adverse effects (mostly low libido or fatigue, which in my experience can sometimes be reversed or reduced by use of adaptogens, particularly Withania somnifera or Panax ginseng), I have generally found it to be quite safe. It is very slow to work, taking 6-24 months. I always stop it after a maximum of 2 years to reduce any risk that it might increase prostate cancer if taken longer, although this possibility is extremely controversial, and a fair bit of evidence suggests it actually reduces the risk of prostate cancer with longer-term use.15
While taking finasteride, I have patients also take saw palmetto, Urtica dioica (nettle) root, and Cucurbita pepo (pumpkin) seed. All of these have distinctive hormone- and growth factor-modulating effects that can help prevent hormonal BPH from progressing, or slow its progression.16,17 I recommend continuing the combination indefinitely to prevent recurrence after completing the drug course. Pumpkin seeds can of course be eaten, with a recommended dose of 3-6 oz per day.
The Most Serious Cases
Patients who have prostates >100 cc, who have a median lobe (meaning the prostate has grown up into the bladder), who have had 2 or more UTIs or episodes of acute retention, who consistently retain ≥100 mL of urine, who have suffered acute renal failure, or who have mild-to-moderate bladder trabeculation are typically best served by having BPH surgery. Usually in these circumstances, the older transurethral resection of the prostate (TURP) procedure is my recommendation. There are many newer procedures that don’t reduce prostate volume as much; however, in patients who have reached this level of severity, I have found that these newer procedures are rarely effective for more than a few months. It is vital that any patient who has surgery follows up with substantial lifestyle changes and use of saw palmetto, nettle root, and pumpkin seed to prevent recurrence within a couple of years.
Unfortunately, patients with severe bladder trabeculation and hypertrophy may not be served by prostate surgery. Even if the obstruction is relieved, the bladder will not revert to normal and they will still have to catheterize. Several of my patients in this situation have tried using all the natural approaches outlined above, sometimes combined with finasteride or a TURP, but there was no obvious benefit to them. Instead, the goal should be to help them prevent infections related to either chronic intermittent catheterization or an indwelling catheter. In these situations, cranberry and/or blueberry, D-mannose, probiotics, and rotating urinary antiseptic herbs (with periodic breaks) constitute my base protocol, and this approach has been quite effective.
Conclusion
In summary, if all that a naturopathic doctor has to offer a patient with BPH is saw palmetto, then, honestly, they are no more useful than the internet. By assessing their patient’s symptoms more carefully, naturopathic doctors can individualize treatment and make it vastly more effective. It is also crucial to recognize that BPH can cause serious complications, and to not overlook this possibility just because symptoms are apparently mild or just because the patient has seen a urologist who has not done a thorough assessment. This approach has been honed over my 23 years of practice and work with 390 patients with BPH (between 2004 and 2019) and is effective. Naturopathic medicine has a lot to offer patients with BPH, both on its own and in combination with conventional medicine where indicated.
References:
- Yarnell E. Natural Approach to Prostate Conditions, 2nd ed. Wenatchee, WA: Wild Brilliance Press; 2017.
- Gu F. Changes in the prevalence of benign prostatic hyperplasia in China. Chin Med J (Engl). 1997;110(3):163-166.
- Platz EA, Kawachi I, Rimm EB, et al. Physical activity and benign prostatic hyperplasia. Arch Intern Med. 1998;158(21):2349-2356.
- Sebastiano C, Vincenzo F, Tommaso C, et al. Dietary patterns and prostatic diseases. Front Biosci (Elite Ed). 2012;4:195-204.
- Barnard RJ, Koabayashi N, Aronson WJ. Effect of diet and exercise intervention on growth of prostate epithelial cells. Prostate Cancer Prostatic Dis. 2008;11(4):362-366.
- Tan MO, Karabiyik I, Uygur MC, et al. Serum concentrations of sex hormones in men with severe lower urinary tract symptoms and benign prostatic hyperplasia. Int Urol Nephrol. 2003;35(3):357-363.
- Gann PH, Hennekens CH, Longcope C, et al. A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. Prostate. 1995;26(1):40-49.
- Wong SY, Lau WW, Leung PC, et al. The association between isoflavone and lower urinary tract symptoms in elderly men. Br J Nutr. 2007;98(6):1237-1242.
- Brössner C, Petritsch K, Fink K, et al. Phytoestrogen tissue levels in benign prostatic hyperplasia and prostate cancer and their association with prostatic diseases. Urology. 2004;64(4):707-711.
- Moon DG, Jin MH, Lee JG, et al. Antidiuretic hormone in elderly male patients with severe nocturia: a circadian study. BJU Int. 2004;94(4):571-575.
- Tacklind J, Macdonald R, Rutks I, et al. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2012;(12):CD001423.
- Carraro JC, Raynaud JP, Koch G, et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: A randomized international study of 1,098 patients. Prostate. 1996;29(4):231-242.
- Djavan B, Fong YK, Chaudry A, et al. Progression delay in men with mild symptoms of bladder outlet obstruction: A comparative study of phytotherapy and watchful waiting. World J Urol. 2005;23(4):253-256.
- Stewart, KM. The African cherry (Prunus africana): can lessons be learned from an over-exploited medicinal tree? J Ethnopharmacol. 2003;89(1):3-13.
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215-224.
- Sökeland J, Albrecht J. Combination of sabal and urtica extract vs. finasteride in benign prostatic hyperplasia (Aiken stages I to II). Comparison of therapeutic effectiveness in a one year double-blind study. Urologe A. 1997;36(4):327-333. [Article in German]
- Bach D. Placebo-controlled, long-term therapeutic study of a pumpkin seed extract product in patients with micturitions complaints from benign prostatic hyperplasia. Urologe B. 2000;40(5):437-443.
 Eric Yarnell, ND, RH (AHG), a 1996 Bastyr graduate, is a full professor in the Department of Botanical Medicine at Bastyr University. Dr Yarnell is the former chair of Botanical Medicine at SCNM and former editor of the Journal of Naturopathic Medicine. He is the CMO at Northwest Naturopathic Urology and has been focused on men’s health, urology, and nephrology for 18 of his 23 years in practice. He is also Chief Creativity Officer at Wild Brilliance Press, and President of Heron Botanicals. He has authored numerous textbooks, including Natural Approach to Urology and Men’s Health, Natural Approach to Gastroenterology, Clinical Botanical Medicine, and Natural Approach to Prostate Conditions.
Eric Yarnell, ND, RH (AHG), a 1996 Bastyr graduate, is a full professor in the Department of Botanical Medicine at Bastyr University. Dr Yarnell is the former chair of Botanical Medicine at SCNM and former editor of the Journal of Naturopathic Medicine. He is the CMO at Northwest Naturopathic Urology and has been focused on men’s health, urology, and nephrology for 18 of his 23 years in practice. He is also Chief Creativity Officer at Wild Brilliance Press, and President of Heron Botanicals. He has authored numerous textbooks, including Natural Approach to Urology and Men’s Health, Natural Approach to Gastroenterology, Clinical Botanical Medicine, and Natural Approach to Prostate Conditions.