Two Distinct Clinical Entities
Nate Champion, ND
It seems as if more and more individuals are being seen with clinical symptoms associated with adverse reactions to gluten, the structural protein component of wheat, barley, and rye. Often, these patients have already been to see a gastroenterologist, and many have had serologic testing performed for celiac disease (CD), wheat allergy, or both. When the results of their blood work come back negative for antibodies to gluten, these patients are often told that wheat or gluten is not a problem and are offered little other advice. These patients are many times left confused, discouraged, and frustrated without any answers and wonder where to go from here. As NDs, we have known for years that individuals can have problems arising from gluten without having true CD. More recently, our understanding and knowledge of gluten sensitivity (GS) has grown, and research has begun to reveal evidence that GS is a separate clinical entity from CD.1
Wheat Allergy and CD
The 2 best-known illnesses related to gluten exposure are wheat allergy and CD, both of which are mediated by the adaptive immune system, with the reaction to gluten being mediated by T-cell activation in the mucosa of the gastrointestinal tract. However, in wheat allergy the release of chemical mediators (histamine) from mast cells and basophils is triggered by the cross-linking of IgE.2 In contrast, CD is an autoimmune disorder indicated by specific serologic markers, most notably serum tissue transglutaminase autoantibodies. Besides CD and wheat allergy, there are many individuals who experience gluten reactions in which neither autoimmune nor allergic mechanisms are involved, generally defined as GS.3
GS vs CD
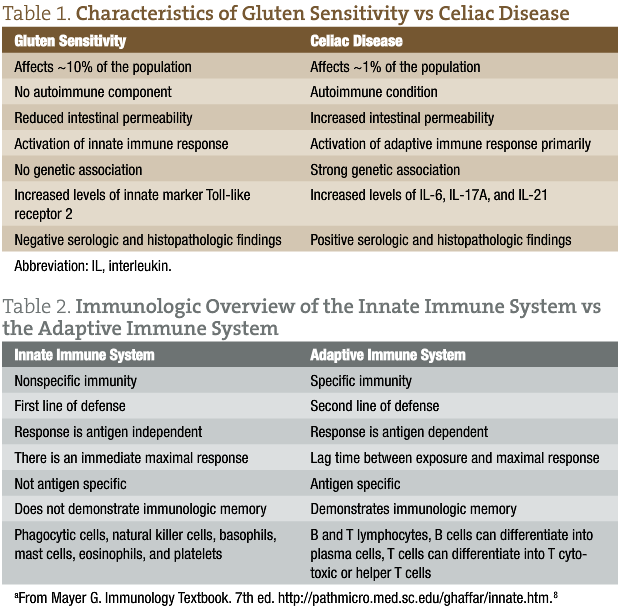
Gluten sensitivity affects approximately 10% of the general population and is considered a diagnosis of exclusion in which patients are considered to be “gluten sensitive” after CD, wheat allergy, and other clinically overlapping diseases (inflammatory bowel disease, type 1 diabetes mellitus, and Helicobacter pylori infection) have been ruled out. In addition, symptoms are triggered by gluten exposure and are alleviated by gluten avoidance. In contrast to CD, which affects approximately 1% of the general population, these adverse symptoms that occur while eating gluten are not followed by the appearance of autoantibodies in the blood or by persistent damage to the small intestine. Table 1 lists characteristics of GS vs CD. A 2011 landmark study3 reported for the first time evidence of different responses in the intestinal mucosa to gluten in GS vs CD. This study showed significantly reduced small intestinal permeability in patients having GS compared with those having CD when tested with a lactulose and mannitol double-sugar probe.
Patients with GS do not seem to present with significant autoimmune or allergic comorbidities, and their serologic test results are negative for common autoantibodies, including transglutaminase IgA. In CD, there is a strong genetic association with the class II major histocompatibility complex proteins. About 95% of patients with CD carry the HLA-DQ2 gene, and the remaining 5% carry the HLA-DQ8 gene. Only about 50% of patients with GS carry either HLA-DQ2 or HLA-DQ8 (a percentage only slightly higher than that in the general population). This suggests that the adaptive immune system has a much more limited involvement in patients with GS and may explain why this condition is not accompanied by significant autoimmune phenomena, as in CD.3 This adaptive immune response in CD has been shown to be triggered by tissue transglutaminase deaminated gluten peptides bound to DQ2 or DQ8. This mucosal recruitment and activation of the helper T cell, subtype 1 (TH1) and TH17 clones and their associated cytokines (interferon γ and interleukin [IL] 17A) contribute to the initiation of tissue damage and disruption of barrier function. In addition to IL-17A, IL-6 and IL-21 (both of which promote differentiation of TH17 cells) are expressed at significantly increased levels in the mucosa of patients with CD but not in those with GS.4 Furthermore, other investigations have demonstrated that IL-17A cytokines are expressed at significantly higher levels in the small intestinal mucosa of patients with CD but not in those with GS. The authors of one study state: “We conclude that GS, albeit gluten-induced, is different from CD not only with respect to the genetic makeup and clinical and functional parameters, but also with respect to the nature of the immune response.”5(p75)
Toll-like receptors (TLRs) have a crucial role in the initiation or maintenance of various immune responses in the innate immune system. One study3 compared the expression of various TLRs using fresh intestinal biopsy specimens from patients with GS or CD. Small intestine expression of TLR2 was significantly increased in patients with GS. TLR1 and TLR4 were generally higher, without reaching clinical significance. Results of this study suggest that the innate immune system has a prevalent role in the pathogenesis of GS, whereas in CD it is the adaptive immune system that has the primary role. More studies are needed to confirm these results, but this finding may help explain the clinical and serologic differences in GS vs CD.
Clinical Implications
So what is the clinical relevance of identifying patients with GS vs those with CD? Is it possible that changes in the innate immune system may precede or accompany the progression of CD and other autoimmune conditions? Based on the current research, it may be too soon to tell. However, investigations have demonstrated that the expression of various TLRs is increased in the small intestinal mucosa of patients with CD, which may lend support to this idea.6 The typical lesions found in the intestines of patients with CD are thought to be mediated by both innate and adaptive immune pathways. Based on the landmark study3 already cited, it seems that GS is associated with prevalent activation of an innate immune response.
When possible, I believe that it is essential to differentiate between GS and CD (tolle causam). It is important for many obvious reasons to understand if you are working with an autoimmune disorder like CD because it can lead to other secondary disorders (thyroid disorders, infertility, etc), not to mention the importance from a genetic and family history standpoint. More and more research has been demonstrating the adverse effects that gluten can have on an increasing percentage of the population. These often include your typical gastrointestinal symptoms, such as gas, bloating, abdominal pain, diarrhea, and constipation. However, many other extraintestinal symptoms involving psychiatric and neurologic manifestations can often present as well. Neurologic manifestations of GS with or without enteropathy are also common. These clinical manifestations can vary, but the most common syndromes involve peripheral neuropathy and cerebellar ataxia. Earlier detection of GS could provide remarkable benefits to patients with neurologic manifestations.7 Finally, Table 2 gives an immunologic overview of the innate immune system vs the adaptive immune system.8
Summary
New research has enhanced our understanding of GS and CD and has shown them to be 2 distinct entities. More double-blind placebo-controlled studies are necessary to further our understanding of GS and to search for specific biomarkers for a proper diagnosis because there is still much we do not know in this regard. The objectives of this article were to inform the clinician about this new research, to further our understanding of these conditions, and to better equip us in the care of our patients. I believe that this information is valuable to our patients as well because it gives credence to what they often experience but rarely have acknowledged by other medical professionals, especially those in allopathic medicine. As NDs, we already have diagnostic tools and specialized testing that we use to detect gluten sensitivities and are in an excellent position to help this growing population of individuals.
 Nate Champion, ND is a graduate of Southwest College of Naturopathic Medicine & Health Sciences, Tempe, Arizona. He is founder and co-owner of Champion Naturopathic Health, LLC, Minnetonka, Minnesota. Dr Champion has a private practice in Minneapolis, Minnesota, with a special focus in digestive disorders, particularly inflammatory bowel disease. In addition to his practice, he regularly reviews research and writes reports on natural medicine for a private health advisory company. For more information, please visit www.championnh.com and www.pih-mpls.com.
Nate Champion, ND is a graduate of Southwest College of Naturopathic Medicine & Health Sciences, Tempe, Arizona. He is founder and co-owner of Champion Naturopathic Health, LLC, Minnetonka, Minnesota. Dr Champion has a private practice in Minneapolis, Minnesota, with a special focus in digestive disorders, particularly inflammatory bowel disease. In addition to his practice, he regularly reviews research and writes reports on natural medicine for a private health advisory company. For more information, please visit www.championnh.com and www.pih-mpls.com.
References
1. Jackson JR, Eaton WW, Cascella NG, Fansano A, Kelly DL. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity [published online ahead of print August 30, 2011]. Psychiatr Q. doi:10.1007/s11126-011-9186-y. Medline:21877216
2. Tanabe S. Analysis of food allergen structures and development of foods for allergic patients. Biosci Biotechnol Biochem. 2008;72:649-659.
3. Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:e23. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3065425/?tool=pubmed. Accessed November 17, 2011.
4. Castellanos-Rubio A, Santin I, Irastorza I, et al. TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity. 2009;42:69-73.
5. Sapone A, Lammers KM, Mazzarella G, et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2009;152:75-80.
6. Szebeni B, Veres G, Dezsofi A, et al. Increased mucosal expression of Toll-like receptor (TLR)2 and TLR4 in coeliac disease. J Pediatr Gastroenterol Nutr. 2007;45(2):187-193.
7. Hernandez-Lahoz C, Mauri-Capdevila G, Vega-Villar J, Rodrigo L. Neurological disorders associated with gluten sensitivity. Rev Neurol. 2011;53(5):287-300.
8. Mayer G. Immunology Textbook. 7th ed. http://pathmicro.med.sc.edu/ghaffar/innate.htm. Accessed November 16, 2011.