Gurdev Parmar, ND, FABNO
With so much information to keep up to date with in a family practice, it is my intention to keep the general-practice ND current with some of the latest relevant oncology research. This article will review colorectal cancer (CRC) literature presented at this year’s American Society of Clinical Oncology (ASCO) meeting. Specifically, the review will cover what CRC is, how common it is, what constitutes a good screening program, what signs and symptoms to watch for, and how to properly work up and follow the CRC patient.
Incidence and Surveillance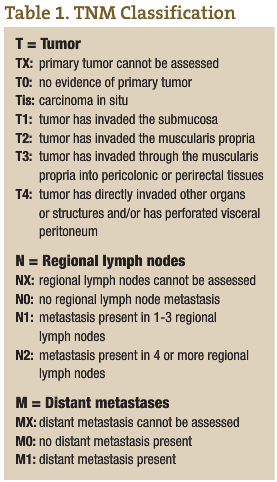
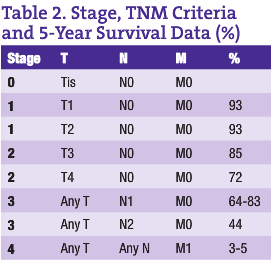
CRC is the third most common cancer in both men and women in Canada and the U.S. (American Cancer Society, n.d.; Casciato and Lowitz, 2000). There are two to three times as many cases of colon cancer as there are of rectal cancer. With a lifetime incidence of up to 5% for Canadian and U.S. populations, it is absolutely imperative that each ND institutes a regular screening program for CRC in his or her practice. Unfortunately, the majority of patients who have CRC have node positive or metastatic disease at the time of diagnosis. In fact, up to 40% of CRC patients have Stage 4 disease at the time of diagnosis (see Tables 1 and 2 for tumor, node, metastasis (TNM) classification and five-year survival rates for the various stages of CRC) (American Cancer Society, n.d.; Casciato and Lowitz, 2000; Li et al., 2007; McMurrick et al., 2006). Therefore, early diagnosis through a coordinated screening program is one way NDs can most significantly impact this patient population.
Prognostic Factors
The five-year survival rate for colorectal cancer is largely stage-dependent (see Table 2), with a vast difference in survival rates between the various stages. For early stage CRCs (Stages 1 and 2), surgical excision gives an excellent outcome if the tumors are localized and clear margins have been achieved. Five-year survival rates of up to 93% have been shown in some studies. However, when it comes to node positive (Stage 3) CRC patients, there is a significant risk for recurrence, even with surgical removal of the primary tumor and adjuvant chemotherapy. As expected, Stage 4 CRC has the poorest prognosis.
With the more recent addition of anti-VEGF and anti-EGFR monoclonal antibodies with standard CRC chemotherapy (FOLFOX and FOLFIRI being the gold standards today), a 3%-5% improvement to five-year survival rates in Stage 4 CRC can be attained (McMurrick et al., 2006; Goldberg, 2005; Benson, 2006; Goldberg, 2006). Many more studies were presented at ASCO with the goal of determining the best way to administer these combination regimens in metastatic disease. The mood remains optimistic with the newer monoclonal agents on board.
 Etiology
Etiology
CRC is most common in western countries (particularly Canada, U.S., Australia and New Zealand), and is very rare in India and South America. In fact, there is a ten-fold difference between those countries with the highest and lowest incidences, suggesting that environmental factors such as diet are involved (Casciato and Lowitz, 2000). A list of some of the most well-accepted etiological factors in the development of CRC includes:
- Polyps (subset of polyps have cancer potential)
- Diet
- High calorie intake (elevated BMI, elevated IGF-1, etc.)
- Low fiber intake
- Low levels of calcium, vitamins A, C, D,E and B-carotene
- High alcohol consumption
- Inflammatory bowel diseases
- Ulcerative colitis (accounts for 1% of CRCs)
- Crohn’s disease increases risk 1.5 to 2 times
- Smoking (increases risk of small and large adenomas)
- Genetics
- Family history found in 15% of patients with CRC (this will include early life environment, of course)
- Environmental factors, including lead, asbestos, chlorine and others
- Infections such as HPV and E. coli
 Screening
Screening
Annual physical examinations are recommended for all at-risk patients (see Table 3). The exam should include a digital rectal examination (DRE) and fecal occult blood test (FOBT) on all patients older than 40 years of age. An astonishing majority of rectal cancers are found with DRE. For patients falling into the higher risk categories, it may be prudent to start screenings as early as 30 years of age. Preventive health and the time taken by NDs to discern the root cause of the presenting illness is a significant benefit of naturopathic medicine. Careful history taking for changes in bowel habits and unexplained weight loss, or a simple CBC aid in early detection. The most commonly accepted recommendations tend to be more conservative and are summarized in Table 3.
Clinical Presentation
It is vital that our doctors recognize the enormous variation in the clinical presentation of CRC. Many patients remain asymptomatic until the advanced stages of their disease and will only be identified by screening (American Cancer Society, n.d.; Casciato and Lowitz, 2000; McMurrick et al., 2006). Any patient older than age 40 with rectal bleeding should be recommended for colonoscopy regardless of etiology. If the bleeding is due to hemorrhoids, a FOBT should be collected after the bleeding has stopped. For patients younger than age 40, further investigation is warranted, especially if there is a family history of CRC and/or if there are any of the following suspicious symptoms:
- bleeding that is not bright in nature (“dark”)
- change in bowel habits (unusual to the patient based on his or her history)
- unexplained weight loss (usually quite marked and relatively fast)
- abdominal pain
- mucous discharge
Patients presenting with these symptoms should be referred for further assessment, and followed up annually for a thorough examination. In my practice I have noted that many CRC patients present with red herring symptoms, including fatigue, low back pain, alternating stools (often leading to the infamous IBS diagnosis) or night sweats. Any patient with chronic iron deficiency anemia should have a FOBT.
Prevention
CRC preventive approaches with substantial evidence and general consensus:
Diet
- <2,500kcal per day for men; <2,000 kcal per day for women
- Reduce dietary animal fats (<25% of total energy as fat)
- >5 portions per day of a variety of vegetables and fruit year-round
- At least 25-30g fiber per day, especially if at high risk of CRC
- Dietary calcium intake of 1,000-1,200mg per day
- Adequate anti-oxidant intake in daily regimen
Healthy lifestyle
- Physical activity 4-7 days per week
- Adequate, not excessive, sun exposure
- Restrict alcohol intake
- No smoking
Cancer Prevention
Selenium supplements, aspirin and other non-steroidal anti-inflammatory drugs (NSAIDS), and selective COX-2 inhibitors have been found to prevent the formation of CRC from polyps (McMurrick et al., 2006), which illustrates the importance of antioxidant supplementation and inflammation management.
Monitoring a CRC Patient
In addition to the conventional oncology team monitoring a CRC patient, there are additional tests that the ND can have performed. A few blood tests are officially approved for their use as “monitoring biomarkers” in CRC. Biomarkers are defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic or pathologic responses to a therapeutic intervention” (Jessup et al., 1993). These tests allow the ND to monitor the progression of CRC quite well when annual surveillance returns to the primary care provider:
- Carcinoembryonic Antigen (CEA) (Mc-Murrick et al., 2006; Wiggers et al., 1986; Kitadai et al., 1996; Jessup et al., 1993; Wanebo et al., 1978)
- CA 19-9 (Haas et al., 2008)
- Circulating Tumor Cells (CTCs) (Carden et al., 2008; Meropol et al., 2007; Cohen et al., 2008)
CEA and CA 19-9 are the most commonly used biomarkers of CRC. The use of CTCs is more recent. Studies have proven CTCs to be a strong, independent predictor of overall survival and progression-free survival. As an adjunct to current standard testing methods, CTC assessment provides a more complete picture of patient prognosis. Other markers can also be useful in monitoring CRC patients, but are not yet approved for such use. They include body mass index (BMI), highly sensitive C-reactive protein and IGF-1.
The ND in family practice can play a significant role in CRC care, including primary prevention, surveillance, monitoring and integrative treatment. With the high prevalence of CRC in our society, screening is warranted. An annual screening program will allow more patients to be detected in Stage 1 or 2, which will result in significantly improved outcomes. The ND also has an essential role in teaching preventive strategies against CRC, as there is an abundance of literature in this area. Lastly, several CRC biomarkers can be used by the ND to monitor the progression of CRC patients and their treatments. NDs have a significant contribution to make in the fight against CRC. The amount of research in the area of integrative oncology has exploded in the recent past. With the addition of newer technologies in diagnosis, staging and treatment, the future of CRC care looks promising.
The Oncology Association of Naturopathic Physicians (OncANP) was founded in 2004 to enhance the quality of life of people living with cancer through both increasing the collaboration between NDs working with patients with cancer and integrating naturopathic practice into medical oncology care. With its co-organization, the American Board of Naturopathic Oncology (ABNO), the OncANP has set standards, instituted testing and now credentials Fellows in naturopathic oncology. The OncANP welcomes all NDs interested in improving their knowledge and ability to work with oncology patients. For more information see www.OncANP.org.
 Gurdev Parmar, ND, FABNO cofounded Integrated Health Clinic in 2000, one of the largest integrated health care facilities in Canada. Dr. Parmar focuses in the treatment of cancer, with a fellowship with the ABNO. He is a member of the ASCO and the OncANP. He lectures regularly for the Canadian Cancer Society and other cancerrelated organizations. Dr. Parmar founded The HELP Foundation, which after the tsunami of 2004 built an Integrated Health Clinic on the island of Kho Khao, Thailand (www.thehelpfoundation.ca). He is licensed in both British Columbia and Arizona. He serves as cochair of the advisory committee on Disaster and Pandemic Preparedness for the province of B.C. In his spare time, Dr. Parmar is a competitive tennis player and enthusiastic Canuck fan. His wife and clinic partner, Karen, and his two sons, Seth and Devan, keep his life balanced and whole.
Gurdev Parmar, ND, FABNO cofounded Integrated Health Clinic in 2000, one of the largest integrated health care facilities in Canada. Dr. Parmar focuses in the treatment of cancer, with a fellowship with the ABNO. He is a member of the ASCO and the OncANP. He lectures regularly for the Canadian Cancer Society and other cancerrelated organizations. Dr. Parmar founded The HELP Foundation, which after the tsunami of 2004 built an Integrated Health Clinic on the island of Kho Khao, Thailand (www.thehelpfoundation.ca). He is licensed in both British Columbia and Arizona. He serves as cochair of the advisory committee on Disaster and Pandemic Preparedness for the province of B.C. In his spare time, Dr. Parmar is a competitive tennis player and enthusiastic Canuck fan. His wife and clinic partner, Karen, and his two sons, Seth and Devan, keep his life balanced and whole.
References
American Cancer Society: www.cancer.org
Casciato DA and Lowitz BB: Manual of Clinical Oncology (4th ed). Philadelphia, 2000, Lippincott, Williams and Wilkins.
Li M et al: Colorectal cancer or colon and rectal cancer? Clinicopathological comparison between colonic and rectal carcinomas, Oncology 73(1-2):52-57, 2007.
McMurrick P et al: Bowel cancer – guide for the GP, Aust Fam Physician 35(4): 192-197, 2006.
Goldberg RM: Advances in the treatment of metastatic colorectal cancer, Oncologist 10(Suppl 3):40-48, 2005.
Benson AB III: New approaches to the adjuvant therapy of colon cancer, Oncologist 11(9):973-980, 2006.
Goldberg RM: Therapy for metastatic colorectal cancer, Oncologist 11:981-987, 2006.
Wiggers T et al: Prognostic significance of CEA immunoreactivity patterns in large bowel carcinoma tissue, Br J Cancer 54(3):409-414, 1986.
Kitadai Y et al: Regulation of carcinoembryonic antigen expression in human colon carcinoma cells by the organ microenvironment, Am J Pathol 149(4):1157-1166, 1996.
Jessup JM et al: Carcinoembryonic antigen: enhancement of liver colonisation through retention of human colorectal carcinoma cells, Br J Cancer 67(3):464-470, 1993.
Wanebo HJ et al: Preoperative carcinoembryonic antigen levels as a prognostic indicator in colorectal cancer, N Engl J Med 299(9):448-451, 1978.
Carden CP et al: Validated and qualified biomarkers critical to optimizing anticancer drug development: potential roles for the study of circulating tumor cells, J Clin Oncol (ASCO Educational Book); 166-170, 2008.
Meropol NJ et al: Circulating tumor cells (CTC) predict progression free (PFS) and overall survival (OS) in patients with metastatic colorectal cancer, J Clin Oncol 25(Suppl 18):S4010, 2007.
Cohen SJ et al: Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer, J Clin Oncol 26(19):3213-21, 2008.
de Haas RJ et al: Tumor marker kinetics: better than imaging to assess response to chemotherapy?, J Clin Oncol 26(No 15S): S4072, 2008.


