Erica Peirson, ND
Every specialty of medicine needs to understand the role that thyroid hormone plays on the organ system in which they specialize. Neurology is just 1 example. This is due to the fact that thyroid hormone, unlike any other hormone in the body, affects every single cell of every organ system. The implications of hypothyroidism, overt or subclinical, on the body are vast.
The brain is one organ of the body that is particularly sensitive to thyroid hormone function. There is much research in the area of thyroid function and its connection to neurological disorders. A sense of urgency exists for many practitioners and researchers who are working hard to increase awareness of the changes to the current dominant paradigm for thyroid care – changes that are desperately needed worldwide. In this article, I will start by reviewing basic thyroid physiology. I will continue by reviewing the effects of thyroid hormone on embryonic brain development and cognition throughout childhood, into adulthood and senescence. Lastly, the many neurological conditions rooted in thyroid hormone dysfunction will be discussed.
Thyroid Physiology
The biggest message I want readers to take home from this overview is that thyroid hormone function is complicated, that it involves so much more than just a TSH and T4 level. It’s a multi-step process in which every step should be considered, especially when symptoms are present. One area of physiology that’s often missed is the role of deiodinase enzymes.
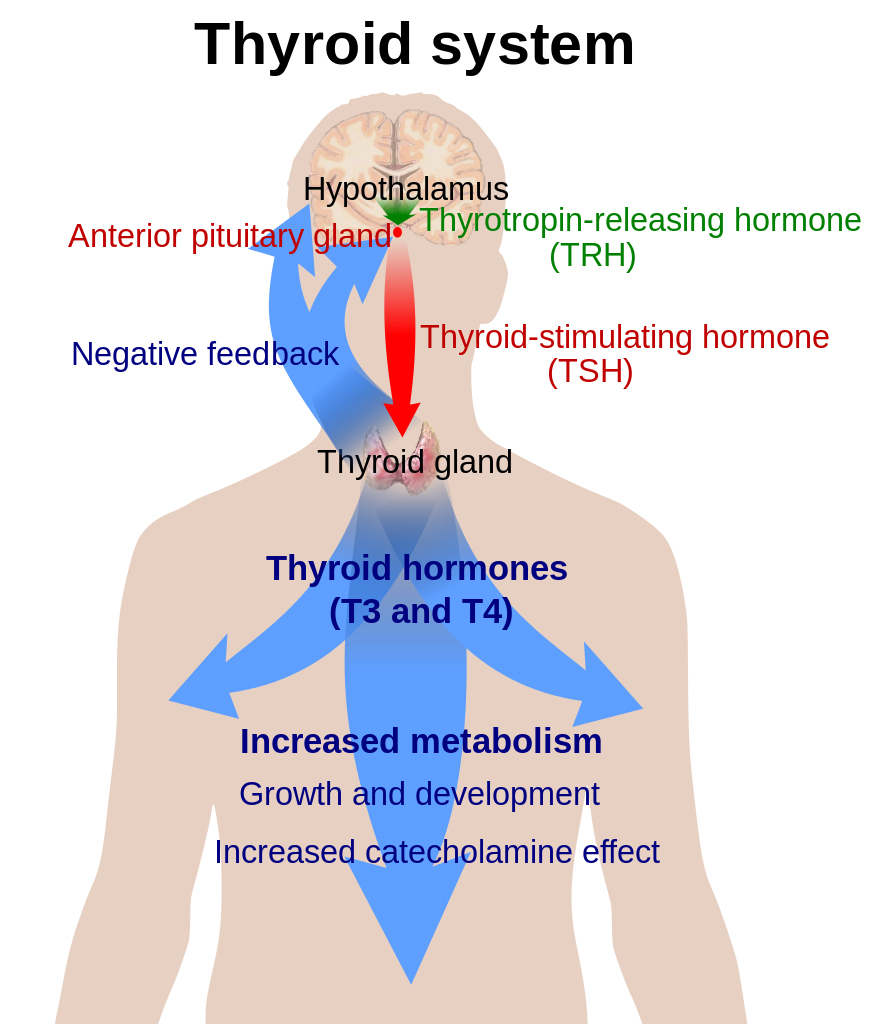
Thyroid hormone function begins with the hypothalamus, which secretes thyrotropin-releasing hormone (TRH). The pituitary gland is triggered by TRH to secrete thyroid-stimulating hormone (TSH). TSH then enters the bloodstream and reaches the thyroid gland where it docks with receptors on the surface of the follicular cells of the thyroid gland. Each receptor then sends signals into the follicular cell that triggers a cascade of events resulting in endocytosis of thyroglobulin from the colloid. Thyroxine (T4) and triiodothyronine (T3) are enzymatically cleaved from thyroglobulin before they are released, in a ratio of 11:1, from the vacuole via exocytosis into the bloodstream, where T4 and T3 begin their journey.
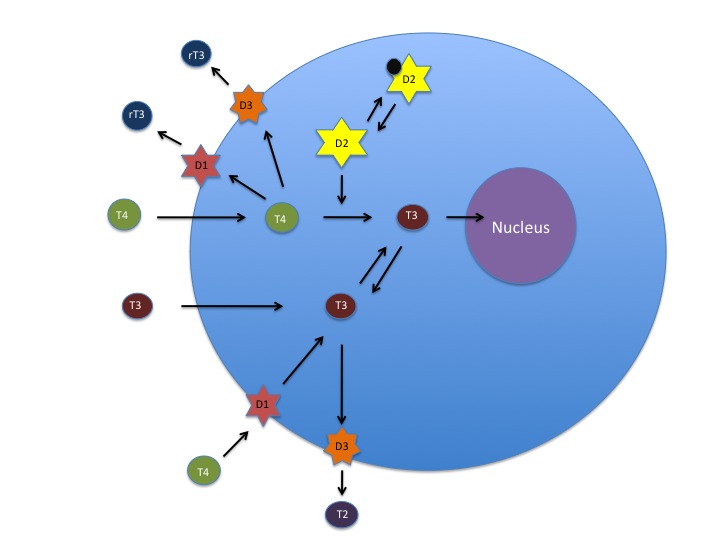
T4 and T3 have different roles within this system. T4 is considered a prohormone, in that it needs to be activated in order to be used. The active form of thyroid hormone is T3. T4 is so named because it has 4 molecules of iodine attached to it. In order for it to be used by the cell, 1 of those iodine molecules must be removed. This is carried out by deiodinase enzymes that are found in every cell of the body. There are several forms of this enzyme – deiodinase 1, 2 and 3 (D1, D2 and D3). Deiodinase enzymes can either activate or inactivate thyroid hormone. D1 can remove an iodine molecule from the inner or outer tyrosine ring of T4; D2 can only remove an iodine from the outer ring; and D3 can only remove an iodine from the inner ring. Removal of different molecules of iodine results in a form of T3 with 2 very different shapes. One is active T3 hormone (made by D2), and the other is reverse T3 (made by D3), which does not function to “turn on” the cell, and can even block active T3 from entering the cell. These different forms of T3 are very important to recognize clinically. A patient with an elevated reverse T3 (rT3) and normal TSH is experiencing hypothyroidism just as much as a patient with a low T4 and elevated TSH.
Deiodinase 1,2 and 3 regulate intracellular levels of T3 and T4. Deiodinase 2 converts T4 to T3, Deiodinase 3 deactivates T4 and T3, and Deiodinase 1 activates T4 to T3 or deactivates it. Ubiquitination regulates Deiodinase 2 activity through deactivation. (Source: Betsy Smartt)
Embryonic Development/Childhood
Some would argue that thyroid hormone is more a hormone of development than of metabolism. It does take on different roles at different stages of life. During embryonic development, the mother’s own thyroid hormone function plays the biggest role in the first stages of development immediately after conception, but is important in all stages of gestation. By 12 weeks gestation, the thyroid gland in the fetus is fully formed but relies on a properly functioning hypothalamic-pituitary axis. Brain development begins very early in gestation and is dependent on the mother’s level of T4 production. By week 5 of gestation, the neural tube is already closing, and by week 6 the brain is beginning to divide into the 5 regions of the brain that will eventually differentiate into recognizable regions of the adult brain (telencephalon, diencephalon, mesencephalon, metencephalon, and myelencephalon).1 Brain development is highly dependent on the action of T3, the active form of thyroid hormone, for myelin formation, cell differentiation, cell migration, cell signaling,2 and SHH (Sonic Hedgehog) signaling.3
Deficiencies in thyroid hormone during this critical time of development can have significant cognitive and behavioral effects. SHH is a protein that is highly involved in the proper development of many fetal organs, particularly the brain. There is also some evidence that it is involved in the function of the postnatal brain as well.4 Given that SHH is highly influenced by thyroid hormone, more research is needed in the connection between thyroid hormone, SHH, and brain development. However, a great deal of evidence exists supporting the negative impact maternal thyroid imbalance (hypo and hyper) has on the development of the fetal brain.5-8 Neurological conditions detected during infancy and childhood that have been linked to aberrant maternal thyroid hormone levels, as well as suboptimal thyroid hormone function during infancy, include autism, ADD/ADHD, Down syndrome, and seizure disorder.9 Although studies exist supporting this, one problem remains: To my knowledge, there are currently no studies assessing reverse T3 levels in children with any of these conditions. These are desperately needed.
Autism
No one can argue that we’re in the midst of a staggering epidemic of autism. The Centers for Disease Control and Prevention (CDC) estimates that as of March 2014, 1 in 68 children were diagnosed with autism. That’s a drastic 30% increase from the 2012 estimate of 1 in 88.10 The rise in the number of children receiving this diagnosis cannot be attributed simply to increased awareness and changes in diagnostic criteria. Autism due to undetected maternal hypothyroidism is preventable.
The most recent study we have connecting maternal thyroid function to childhood autism was published in March of this year. Researchers from NYU used data from the Finnish Prenatal Study of Autism (FiPS-A), collected from 1987-2005 on mothers of children with autism. They looked at records of 967 “matched case controlled pairs.” What they found was an 80% increase in the risk of autism in children born to mothers who had elevated thyroid peroxidase (TPO) autoantibodies.11 TPO antibodies can create variable thyroid function that can differ from week to week. It’s unclear whether these mothers had low thyroid hormone levels during the first 5 weeks of gestation. What is clear, however, is that TPO antibodies do cross the placenta and can impact thyroid function of the fetus in utero.12 Another study, published in 2013, concludes, “We found a consistent association between severe, early gestation maternal hypothyroxinemia and autistic symptoms in offspring.”13 These researchers defined hypothyroidism as free T4 at less than the fifth percentile of the reference range, with a normal TSH. Physicians who assess thyroid function in a woman of child-bearing age with a TSH only, ignoring symptoms and not acquiring further labs, are potentially contributing to the epidemic of autism.
In addition, many of the symptoms of pediatric hypothyroidism parallel those of autism (Table 1).
Table 1. Symptoms of Both Pediatric Hypothyroidism & Autism
| Low muscle tone |
| Feeding issues |
| Developmental delay |
| Sleep disturbances |
| Frequent infections |
| Delayed speech and cognition |
| Delayed growth/short stature |
| Constipation |
| Gastric reflux |
| Dry skin |
| Delayed dentition |
| Protruding tongue |
Our current epidemic of autism coinciding with our current epidemic of hypothyroidism is no coincidence. Women who experience genetic predisposition to hypothyroidism, nutrient deficiencies, exposure to the vast amount of endocrine-disrupting chemicals in our environment, and stress are all at risk for subclinical hypothyroidism, as reported by researchers from the University of Texas as far back as 2007.14 Those same researchers, together with researchers from Spain and NYU, continued their investigation into the role that hypothyroidism plays in autism, and stated, “The morphological alterations of cortical lamination observed in mouse models of developmental hypothyroidism prompted the recognition that these experimental changes resembled the brain lesions of children with autism.”15 Physicians must learn to see beyond a simple TSH lab and recognize the subtle symptoms of hypothyroidism for the sake of future generations of children.
Case Study 1
Case: 5-year-old boy with autism
TSH at birth: 30 mIU/L (cut-off for diagnosis of congenital hypothyroidism in his state of birth = 33 mIU/L)
Treatment: None. Patient was discharged to return home.
Health during infancy: Severe reflux, many “sick” visits, delayed growth and development
TSH at 2.5 years old: 11 mIU/L
Treatment at 2.5 years old: Started on levothyroxine. (He’s now on dessicated thyroid.)
Result: Reflux gone on the first day; increased eye contact; abdomen no longer bloated; skin no longer dry; hair shinier; dark circles under eyes gone
Current health: Diagnosed with autism; non-verbal; short stature; bone age at 5 years old = 3.5 years
ADHD
Attention-deficit/hyperactivity disorder (ADHD) is characterized by lack of focus, hyperactivity, and impulsiveness. It, too, has potential roots in maternal thyroid dysfunction. A 2011 study from Denmark states, “children of TPOAb-positive mothers were at a higher risk of attention deficit/hyperactivity problems.”16 Recall that TPO antibodies cross the placenta. This begs the question of thyroid function in actual children with ADHD, and not just maternal levels in utero. In 2013, researchers in Spain found that, “Despite being within the normal range, high TSH concentrations are associated with a lower cognitive function and high TSH and low free T4 with ADHD symptoms in healthy preschoolers.”17 Once again, TSH was within normal range, but high within the range for children with ADHD symptoms.
Trisomy 21
My work to help children with Trisomy 21 (T21) is what led me to learn about the function of thyroid hormone and the developing brain. The impact that optimal thyroid hormone function can have on a child with T21 is profound. Similar to autism, the symptoms of T21 are nearly identical to those of neonatal and pediatric hypothyroidism (Table 1).
Several research centers are working hard to uncover the connection between hypothyroidism and T21, given these very obvious similarities. Currently, however, no research is underway to assess reverse T3 levels in infants with T21 compared to typical infants. It is normal for a newborn infant to have rT3 levels that are much higher than adult levels.18 It serves the infant by protecting them from active T3 levels in the mother’s body that are meant for the mother and not for the small body of the growing infant. Only 1 study exists that reports on rT3 levels in typical children, which states, “high serum rT3 concentration in the newborn becomes comparable to that in the normal adult by 9-11 days of neonatal life.”19
In my practice, I find a majority of infants as old as 6, 10 and 18 months old with T21 to have an elevated rT3, sometimes as high as 48 ng/dL and even 70 ng/dL (reference range = 8-25 ng/dL). In addition, when the appropriate medication, liothyronine (synthetic T3), is given to these infants, their development and health often very quickly and dramatically improves.
Researchers at the University of Amsterdam studied thyroid hormone levels in 97 newborns and infants with Down syndrome (DS) and concluded, “These findings suggest that as a group DS infants have a novel type of persistent mild congenital hypothyroidism, presumably of thyroidal origin.”20 “Novel” implies a unique form of hypothyroidism. However, it’s not unique to infants with T21 and is rooted in an elevated rT3, which many other people without T21 experience. In addition, children with T21 commonly experience low zinc levels, which have been linked to hypothyroidism.21 Simply supplementing with zinc can improve thyroid function.22
One of the biggest physiological obstacles faced by children with T21 is oxidative stress.23 This process can have a big impact on proper thyroid hormone function. A study done in Norway in 2000 detected low levels of selenium in adults with T21, in addition to elevated TSH and low free T4 levels. They state, “Our results support the suggestion that thyroid hypofunction in patients with Down’s syndrome in some way is linked to the low serum levels of selenium found in these patients.”24 In fact, the enzymes involved in the synthesis and activation of thyroid hormones – deiodinases – are seleno-proteins. It astounds and saddens me that these adults with elevated TSH and low free T4 were even found and had most likely gone their whole lives without proper thyroid treatment, a treatable form of irreversible mental retardation.
The climate of health care for parents of children with Down syndrome, who have concerns for the health of their children, often includes, “That’s normal for Down syndrome” – so much so that symptoms of hypothyroidism are often overlooked because they are expected. Researchers at the University of Oklahoma set out to determine the adherence of physicians to even the basic guidelines set by the American Academy of Pediatrics for thyroid screening in children with T21. They found that adherence to basic guidelines was as low as 13% in Oklahoma, and only as high as 45% in Nebraska.25
Seizures
The etiology of seizures is still largely unknown. Medical management often includes pharmaceuticals that can have fairly detrimental side effects, including a negative impact on thyroid function.26 In 2013, researchers found a significant increased risk of febrile seizures in infants diagnosed with hypothyroidism after birth.27 In addition, there is a great deal of research assessing the role that the GABAergic system has in the etiology of seizures.28-31 Given this evidence, one cannot ignore the impact that thyroid hormone plays on the GABAergic system of the brain. A rat study from 2013 makes this statement: “These results indicate that the development of GABAergic terminals and the excitatory to inhibitory maturation of GABA signaling are strongly dependent on TH [thyroid hormone].”32 More evidence of the strong connection between thyroid hormones and GABA comes from animal researchers in Germany who state, “Early in neocortical network development, triiodothyronine (T3) promotes GABAergic neurons’ population increase, their somatic growth and the formation of GABAergic synapses.”33 While the connection between thyroid hormone and seizures has not been fully established, the justification for future research is evident to uncover whether the connection is causation versus correlation.
Conclusion
Physicians should educate themselves about the entire process of thyroid hormone function. Focusing on learning the root causes of dysfunction associated with each step will help guide the physician to being able to test for and address these root causes for their patient. Some root causes of thyroid dysfunction, among others, include adrenal dysfunction, hypopituitarism, zinc deficiency, iron deficiency, selenium deficiency, and iodine deficiency.
The brain is very plastic. It was once widely accepted that we are only born with the number of neurons we will ever have and that neurogenesis is not possible after birth. This view is slowing changing and being disproven by scientific studies and clinical experience. In the presence of hypothyroidism, however, this plasticity is less likely to occur, given the huge obstacle that thyroid hormone dysfunction can have to proper brain function. When helping patients with any neurodegenerative condition, physicians should maintain a high level of suspicion for hypothyroidism and conduct thorough thyroid lab work to rule out hypothyroidism as a potential contributing cause.
 Erica Peirson, ND, is a naturopathic physician and the mother of a 7-year-old son with Mosaic Down Syndrome who experienced undiagnosed hypothyroidism as an infant. She is dedicated to helping other children avoid this potentially devastating start in life. She sees patients privately at the Peirson Center for Children, and is the executive director of Down Syndrome OPTIONs. She writes and lectures whenever possible to help spread awareness to parents and physicians that the symptoms of Down syndrome are treatable through proper treatment and detection of hypothyroidism, among other things. Her knowledge gained working with these children has aided her in helping other children with special needs, including those with autism.
Erica Peirson, ND, is a naturopathic physician and the mother of a 7-year-old son with Mosaic Down Syndrome who experienced undiagnosed hypothyroidism as an infant. She is dedicated to helping other children avoid this potentially devastating start in life. She sees patients privately at the Peirson Center for Children, and is the executive director of Down Syndrome OPTIONs. She writes and lectures whenever possible to help spread awareness to parents and physicians that the symptoms of Down syndrome are treatable through proper treatment and detection of hypothyroidism, among other things. Her knowledge gained working with these children has aided her in helping other children with special needs, including those with autism.
References:
- Levene MI. Fetal and Neonatal Neurology and Neurosurgery. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2009.
- Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95-122.
- Desouza LA, Sathanoori M, Kapoor R, et al. Thyroid hormone regulates the expression of the sonic hedgehog signaling pathway in the embryonic and adult mammalian brain. Endocrinology. 2011;152(5):1989-2000.
- Álvarez-Buylla A, Ihrie RA. Sonic hedgehog signaling in the postnatal brain. Semin Cell Dev Biol. 2014;33:105-111.
- Morreale de Escobar G, Escobar del Rey F, Ruiz-Marcos A. (1983). Thyroid hormone and the developing brain. In: Dussault JH, Walker P, eds.Congenital Hypothyroidism. New York, NY: Marcel Decker, Inc; 1983:85-126.
- Alkemade A. Thyroid hormone and the developing hypothalamus. Front Neuroanat. 2015;9:15.
- Zoeller RT. Transplacental thyroxine and fetal brain development. J Clin Invest. 2003;111(7):954-957.
- Opazo MC, Gianini A, Pancetti F, et al. (2008) Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology. 2008;149(10):5097-5106.
- Andersen SL, Olsen J, Laurberg P. Foetal programming by maternal thyroid disease. Clin Endocrinol (Oxf). 2015 Feb 12. [Epub ahead of print]
- Centers for Disease Control and Prevention. CDC estimates 1 in 68 children has been identified with autism spectrum disorder. March 27, 2014. CDC Newsroom. http://www.cdc.gov/media/releases/2014/p0327-autism-spectrum-disorder.html. Accessed April 12, 2015.
- Brown AS, Surcel HM, Hinkka-Yli-Salomäki S, et al. Maternal thyroid autoantibody and elevated risk of autism in a national birth cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:86-92.
- Dallas JS. Autoimmune thyroid disease and pregnancy: relevance for the child. Autoimmunity. 2003;36(6-7):339-350.
- Román GC, Ghassabian A, Bongers-Schokking JJ, et al. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol. 2013;74(5):733-742.
- Román GC. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci. 2007;262(1-2):15-26.
- Berbel P, Navarro D, Román GC. An evo-devo approach to thyroid hormones in cerebral and cerebellar cortical development: etiological implications for autism. Front Endocrinol (Lausanne). 2014;5:146.
- Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG. 2014;121(11):1365-1374.
- Alvarez-Pedrerol M, Ribas-Fito N, Torrent M, et al. TSH concentration within the normal range is associated with cognitive function and ADHD symptoms in healthy preschoolers. Clin Endocrinol (Oxf). 2007;66(6):890-898.
- Brown RS. Disorders of the Thyroid Gland in Infancy, Childhood and Adolescence. Updated March 21, 2012. In: www.thyroidmanager.org. South Dartmouth, MA: Endocrine Education, Inc. Available at: http://www.thyroidmanager.org/chapter/disorders-of-the-thyroid-gland-in-infancy-childhood-and-adolescence/. Accessed April 12, 2015.
- Chopra IJ, Sack J, Fisher DA. Circulating 3,3′, 5′-triiodothyronine (reverse T3) in the human newborn.J Clin Invest. 1975;55(6):1137-1141.
- van Trotsenburg AS, Kempers MJ, Endert E, et al. Trisomy 21 causes persistent congenital hypothyroidism presumably of thyroidal origin. Thyroid. 2006;16(7):671-680.
- Napolitano G, Palka G, Lio S, et al. Is zinc deficiency a cause of subclinical hypothyroidism in Down syndrome? Ann Genet. 1990;33(1):9-15.
- Bucci I, Napolitano G, Giuliani C, et al. Zinc sulfate supplementation improves thyroid function in hypozincemic Down children. Biol Trace Elem Res. 1999;67(3):257-268.
- Jovanovic SV, Clements D, MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome.Free Radic Biol Med. 1998;25(9):1044-1048.
- Kanavin OJ, Aaseth J, Birkrtvedt GS. Thyroid hypofunction in Down’s syndrome: is it related to oxidative stress? Biol Trace Elem Res. 2000;78(-3):35-42.
- Fergeson MA, Mulvihill JJ, Schaefer GB, et al. Low adherence to national guidelines for thyroid screening in Down syndrome. Genet Med. 2009;11:548-551.
- Yilmaz U, Yilmaz TS, Akinci G, et al. The effect of antiepileptic drugs on thyroid function in children. Seizure. 2014;23(1):29-35.
- Andersen SL, Laurberg P, Wu CS, Olsen J. Maternal thyroid dysfunction and risk of seizure in the child: a Danish nationwide cohort study. J Pregnancy. 2013;2013:636705.
- Petroff OA, Rothman DL, Behar KL, Mattson RH. Low brain GABA level is associated with poor seizure control. Ann Neurol. 1996;40(6):908-911.
- Chapter 1, Basic Mechanisms Underlying Seizures and Epilepsy. In: Bromfield EB, Cavazos JE, Sirven JI, eds. An Introduction to Epilepsy [Internet]. West Hartford, CT: American Epilepsy Society; 2006. Available at: http://www.ncbi.nlm.nih.gov/books/NBK2510/. Accessed April 12, 2015.
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8-12.
- Mathews GC. The dual roles of GABA in seizures and epilepsy generate more excitement. Epilepsy Curr. 2007;7(1):28-30.
- Sawano E, Takahashi M, Negishi T, Tashiro T. Thyroid hormone-dependent development of the GABAergic pre- and post-synaptic components in the rat hippocampus. Int J Dev Neurosci. 2013;31(8):751-761.
- Westerholz S, de Lima AD, Voigt T. Thyroid hormone-dependent development of early cortical networks: temporal specificity and the contribution of trkB and mTOR pathways. Front Cell Neurosci. 2013;7:121.