Alex Vasquez, DC, ND, DO, FACN
Steroidal and peptide hormones have significant immunomodulating properties, and a characteristic pattern of disruption is commonly seen in patients with autoimmunity. Relatively simple natural and/or pharmacologic interventions can be used to safely and effectively correct hormonal disturbances, with the dual benefits of improved overall health and the amelioration of autoimmunity. I have coined the term “orthoendocrinology”‡ to describe this technique of addressing numerous disturbances in endocrine function by using the “right hormones,” based on a similar conceptual model as Linus Pauling’s suggestion that health might be optimized by the use of the “right molecules” (orthomolecular medicine, orthomolecular nutrition) rather than endless reliance on the cyclical prescriptions of what he called “toximolecular” substances.
I will keep this overview and application straightforward, simple, and clinically relevant; readers should appreciate that this therapeutic approach of hormonal optimization is only 1 of 7 major areas of intervention in my anti-rheumatic therapeutic approach for addressing the causes of sustained inflammatory responses, formerly called “chronic inflammation.”
This discussion’s focus on hormones/endocrinology is, in fact, the sixth of the 7 areas of intervention for reducing inflammation:
- Food
- Infections
- Nutritional immunomodulation
- Dysfunctional mitochondria
- Sleep, stress, and style of living
- Endocrinology
- Xenobiotic immunotoxicity – recalled by the FINDSEX® acronym1
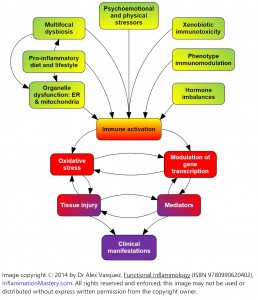
Of course, these interventions are most effective when employed within a comprehensive treatment plan that includes all components of the overall protocol. The colorful graphic (Figure 1) illustrates how these 7 factors coalesce to effect immunodysregulation that underlies states of so-called chronic inflammation, seen at its worse in the clinical manifestation of autoimmunity.
Major Concepts
Hormones affect and are affected by inflammatory status. In this section, I will focus on the 7 hormones of greatest significance to inflammatory/autoimmune homeodynamics. Readers should mentally organize this information in the following way:
- Hormones that are commonly elevated and should be reduced in inflammatory states: Prolactin, Insulin, Estrogen
- Hormones that are commonly deficient and should be increased in inflammatory states: Cortisol, DHEA, Testosterone
Thyroid status is considered separately. Thyroid status is a less potent influence on inflammatory balance when compared to the 6 other hormones listed below; however, occasionally a patient will have severe musculoskeletal inflammation (eg, bilateral adhesive capsulitis, oligoarthritis, proximal myopathy2) that is solely responsive to thyroid hormone monotherapy. I have reviewed the assessment and treatment of hypothyroidism, as well as Hashimoto’s disease, in a small review elsewhere,3 and within a more complete context addressing the entirety of inflammation and assessment and treatment of common autoimmune disorders.4
Hormonal Testing and Treatment in Patients with Autoimmunity
Patients with autoimmune disorders commonly have deficiencies or other imbalances in their hormones, particularly steroid and “sex” hormones, which are potent immunomodulators. The classic pattern which may be expressed incompletely in an individual patient, is that of excess prolactin and estrogen, and insufficient cortisol, testosterone, and DHEA. The role of progesterone seems less clear, with some patients showing normal, excess, or insufficient amounts; obviously, this can be tested in an individual patient (random serum test in men, or Day 21/mid-luteal serum sample from a premenopausal woman).
All testing methods – whether via serum, saliva, or urine – have their individual advantages and disadvantages. Generally however, serum is considered the standard; following this, I prefer polyhormonal assessment with 24-hour urine collections. The last resort is salivary testing, which is the most controversial and least reliable for most hormones. Thyroid autoimmunity and hypothyroidism are both common in autoimmune/inflammatory patients; comprehensive thyroid assessment with TSH, T4, and anti-TPO antibodies can be justified in nearly any rheumatic patient.
Hyperprolactinemia
Besides its obvious role in lactation, prolactin is a polyfunctional hormone that, at the very least, stimulates the liver to produce excess sex hormone-binding globulin (SHBG), which adsorbs sex hormones, rendering them less effective due to reduced bioavailability. The resultant functional hypogonadism deprives the immune system of the modulation/suppression normally effected by these hormones. Beyond its well-known physiologic roles, prolactin is now known to be powerfully proinflammatory.5 Patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) have higher basal and stress-induced levels of prolactin compared with normal controls.6,7 Patients with scleroderma have relatively high prolactin8 and low DHEA.9 Patients with polymyalgia rheumatica have elevated prolactin that correlates with increased symptomatology.10
Prolactin’s effect of increasing hepatic production of SHBG reduces bioavailability of testosterone, thus depriving cells and tissues of testosterone’s potent anti-inflammatory and immunoregulatory properties. Prolactin is routinely measured in serum; high values should be reduced with effective treatment, whether nutritional, botanical, or pharmacologic:
- Thyroid hormone: Hypothyroidism frequently causes hyperprolactinemia that is reversible upon effective treatment of hypothyroidism. Conversely, due to its immunodysregulating effects, elevated prolactin appears capable of inducing hypothyroidism and autoimmune thyroiditis.11 Assessing hyperprolactinemic patients for thyroid disturbances with TSH, T4, T3, and anti-TPO antibodies is strongly advised. Thyroid status should be evaluated in all patients with hyperprolactinemia.
- High-dose pyridoxine: Vitamin B6 (250 mg QD-BID with food) is used by some doctors to lower prolactin, despite the lack of consistently demonstrated benefit in the research literature
- Vitex agnus-castus and other supporting botanicals and nutrients: Vitex lowers serum prolactin in humans12,13 via a dopaminergic effect.14 Vitex (chasteberry) is considered safe for clinical use; mild and reversible adverse effects possibly associated with chasteberry include nausea, headache, gastrointestinal disturbances, menstrual disorders, acne, pruritus, and erythematous rash. No drug interactions are known, but given the herb’s dopaminergic effect, it should probably be used with some caution in patients treated with dopamine antagonists such as the so-called antipsychotic drugs (most of which do not work very well and/or carry intolerable adverse effects15,16). In a recent review, Bone17 stated that daily doses can range from 500 to 2000 mg DHE (dry herb equivalent) and can be tailored to the level of prolactin. Due at least in part to its content of L-dopa, Mucuna pruriens shows clinical dopaminergic activity, as evidenced by its effectiveness in Parkinson’s disease18; up to 15-30 g per day of mucuna have been used clinically, but doses should be based on preparation and phytoconcentration. Ironically, even though tyrosine is the nutritional precursor to dopamine, with evidence of clinical effectiveness (eg, narcolepsy,19 enhancement of memory,20 and cognition21), supplementation with tyrosine appears to actually increase rather than decrease prolactin levels22; therefore tyrosine should be used cautiously, if at all, in patients with systemic inflammation. Furthermore, the finding that high-protein meals stimulate prolactin release23 may partly explain the benefits of vegetarian diets in the treatment of systemic inflammation; since vegetarian diets are comparatively low in protein compared to omnivorous diets, they may lead to a relative reduction in prolactin production due to lack of stimulation.
- Bromocriptine: This agent has long been considered the pharmacologic treatment of choice for elevated prolactin.24 The typical dose is 2.5 mg per day (effective against SLE); gastrointestinal upset and sedation are common. Clinical intervention with bromocriptine appears warranted in patients with RA, SLE, Reiter’s syndrome, psoriatic arthritis, and probably multiple sclerosis and uveitis.5
- Cabergoline: This is a newer dopamine agonist with few adverse effects. The typical dose starts at 0.5 mg per week (0.25 mg twice weekly).26 Several studies have indicated that cabergoline is safer and more effective than bromocriptine for reducing prolactin levels,27 and the dose can often be reduced after successful prolactin reduction, allowing for reductions in cost and adverse effects.28 Although fewer studies have been published supporting the anti-rheumatic benefits of cabergoline vs bromocriptine, the scientific rationale for its use is derived from the success of bromocriptine in various rheumatic/autoimmune diseases. Furthermore, clinicians need to appreciate that prolactin is an active proinflammatory signal within the immune system (functioning in an autocrine/paracrine manner among immunocytes) and that treatment can be implemented and is often effective regardless of serum prolactin levels; if a high serum prolactin is found, it should be treated, whereas a normal serum prolactin does not preclude use of anti-prolactin treatment. Erb et al29 published a case report of a woman with unremitting RA who achieved remarkable clinical improvement using cabergoline, and who was able to reduce her need for other anti-rheumatic drugs using a dose of 0.5 mg per day.
Insulin, Hyperinsulinemia
As a marker for insulin resistance, and thus possibly xenobiotic exposure and mitochondrial dysfunction as well, fasting serum insulin is a surrogate marker for an inflammatory state. Insulin itself shows some anti-inflammatory effects; however, in a clinical context, elevated serum insulin correlates with systemic inflammation and dysmetabolism. Although major medical labs use an insulin reference range of 2.6-24.9 μIU/mL, such a range is clinically ridiculous except for screening for overt pathology; this range should be replaced with the following:
- Optimal: fasting serum insulin <5 μIU/mL
- Mild-moderate insulin resistance: fasting serum insulin 5-15 μIU/mL
- Marked-severe insulin resistance: fasting serum insulin 15 μIU/mL
The goal, when considering serum insulin within the context of alleviating inflammation, is not to lower serum insulin per se, but rather to alleviate the causative insulin resistance. Stated perhaps more plainly, an elevation in fasting serum insulin is indicative of insulin resistance (except in the exceedingly rare case of insulinoma or exogenous insulin administration, which can be distinguished by concomitant measurement of C-peptide). Insulin resistance is a proinflammatory state; thus, our goal is to reduce fasting serum insulin, not because we are targeting insulin per se, but rather because we are measuring serum insulin as a marker for insulin resistance and thus a particular type of metabolic inflammation. First-line therapy for insulin resistance is carbohydrate restriction via a plant-based, low-carbohydrate Paleo diet; exercise and physical activity >60 minutes/day; and vitamin and mineral supplementation, especially vitamin D3, chromium, magnesium, CoQ10, and mixed tocopherols.
Estrogen
Nearly all autoimmune disorders are more common in females than males, and occasionally a menstrual exacerbation is noted, suggesting a possible immunodysregulation by estrogen. Despite the complex and multifaceted nature of the endocrine system, we may safely generalize that the estrogens are immunostimulatory and immunodysregulatory, while androgens are immunosuppressive and immunoregulatory30; thus, elevated estrogen:androgen ratios promote/exacerbate autoimmunity. Furthermore, many chemical/pollutant xenobiotics have estrogen-like effects (“xenoestrogens”) and are consistently associated with induction or exacerbation of autoimmunity. So-called “estrogen-replacement therapy” used in postmenopausal women increases the risk of lupus and scleroderma.31 Men with RA show an excess of estradiol and a decrease in DHEA, and the excess estrogen is proportional to the degree of inflammation.32 Estrogen(s) can be measured in serum and/or 24-hour urine samples. Beyond looking at estrogens from a quantitative standpoint, they can also be qualitatively analyzed with respect to the ratio of estrone:estradiol:estriol, as well as the balance between the “good” 2-hydroxyestrone and the purportedly carcinogenic and proinflammatory 16α-hydroxyestrone. Interventions to lower estrogen levels include the following:
- Weight loss and weight optimization: Excess adiposity and obesity raise estrogen levels, due to high levels of aromatase (the hormone that makes estrogens from androgens) in adipose tissue; weight optimization and loss of excess fat helps normalize hormone levels and reduce inflammation. In overweight patients, weight loss is the means to attaining the goal of weight optimization; the task is not complete until the body mass index is normalized/optimized.
- Avoidance of ethanol: Ethanol stimulates estrogen production, particularly in men
- Surgical correction of varicocele in affected men: Men with varicocele have higher estrogen levels due to temperature-induced alterations in enzyme function in the testes; surgical correction of the varicocele lowers estrogen levels
- “Anti-estrogen diet”: Foods and supplements such as green tea, diindolylmethane (DIM), indole-3-carbinol (I3C), licorice, and a high-fiber, crucifer-based “anti-estrogenic diet” can also be used; monitoring clinical status and serum estradiol will prove or disprove efficacy. Whereas 16α-hydroxyestrone is proinflammatory and immunodysregulatory, 2-hydroxyestrone has anti-inflammatory action33 and has been described as “the good estrogen,”34 due to its anti-cancer and comparatively health-preserving qualities. In a recent short-term study using I3C in patients with lupus, I3C supplementation at 375 mg per day was well-tolerated and resulted in modest treatment-dependent clinical improvement, as well as favorable modification of estrogen metabolism away from 16α-hydroxyestrone and toward 2-hydroxyestrone.35
- Pharmacologic aromatase inhibition: In the early years of my clinical practice, partly under the tutelage of an experienced “integrative” medical physician, I learned to measure serum estradiol in men and administer the aromatase inhibitor anastrozole (1 mg at ³2-3 doses per week) to men whose estradiol level was greater than 32 pg/mL. The Life Extension Foundation36 advocates that the optimal serum estradiol level for a man is 20-30 pg/mL. Clinical studies using anastrozole in men have shown that aromatase blockade lowers estradiol and raises testosterone37; generally speaking, this is exactly the result that we want in patients with severe systemic autoimmunity. Whether using anastrozole, frequency of dosing is based on serum and clinical response. Letrozole is a newer pharmacologic aromatase inhibitor which appears to have slight superiority over anastrozole in terms of anti-estrogen efficacy; however, I am quite convinced that letrozole is also an androgen receptor agonist, and therefore I generally avoid this drug like the plague. Relatedly, the botanical Rhodiola rosea can elevate testosterone and estradiol, making the latter particularly difficult to control. On occasion, we have seen some men make so much testosterone and rapidly convert it to estradiol that they required both anastrozole and letrozole, along with daily licorice, in order to control their testosterone and estradiol levels. Licorice lowers testosterone (the precursor to estradiol in both men and women) within about 4 days of oral administration, whether by standardized capsules or by tea from the root. Of course, anastrozole can be administered to women in 1 mg doses, ranging from once per week to 5-7 times per week, depending on clinical and serologic response. In 2 recent case reports, administration of anti-estrogen medication – either tamoxifen or anastrozole – resulted in clinical improvements in 2 women with dermatomyositis.38
Testosterone
Androgen deficiencies predispose to, are exacerbated by, and contribute to autoimmune/inflammatory disorders. A large proportion of men with SLE or RA have low testosterone39 and suffer the effects of hypogonadism, including fatigue, weakness, depression, slow healing, low libido, and difficulties with sexual performance. Particularly in men, blood samples should be drawn for both free and total testosterone, along with serum estradiol. Since some labs accept ridiculously low levels of testosterone as “within normal limits,” clinicians should not necessarily wait until the patient is pathologically hypogonadal before implementing treatment. Clinicians must appreciate the interrelationship of testosterone with estrogen in order to interpret the patient’s status appropriately and implement proper treatment.
Since testosterone is converted to estradiol by aromatase, a patient with low testosterone and high estradiol is properly treated with aromatase inhibition (eg, anastrozole) rather than testosterone, since administration of anastrozole to men simultaneously lowers estradiol and raises testosterone.
Conversely, low testosterone along with low estrogen indicates the appropriateness of testosterone replacement and/or corrective treatment of the cause of the hypogonadism. The need for co-administration of testosterone and anastrozole is not uncommon, in order to raise testosterone without leading to an iatrogenic increase in estrogen due to “estrogen shunting” by aromatase. Follow-up testing of testosterone and estradiol 4-8 weeks after implementing testosterone replacement is advised to ensure optimal hormone status and that the additional immunoregulatory testosterone is not being shunted into immunodysregulatory estradiol. For the purpose of gently but effectively modulating/lowering estrogen levels, anastrozole is typically administered as 1 mg, 1-4 times per week. The drug appears to have a wide margin of safety, and doses of 1 mg per day are commonly and appropriately used (as one of the safest and most effective treatments against breast cancer) in women with estrogen-responsive breast cancer.
As with any modulation/administration of testosterone and estrogen, follow-up testing of hormones and serum lipids is recommended to ensure the attainment of optimal status and the avoidance of complications, such as suppression of HDL synthesis, which would be expected to have adverse cardiovascular consequences. Assessing serum PSA is a prerequisite to testosterone administration in men. Testosterone therapy improves clinical status and well-being in women with RA.40 Transdermal testosterone creams can be used, with the dose tailored to serum and clinical response.
Cortisol
In physiologic doses, cortisol is immunoregulatory and mildly yet significantly immunosuppressive (“physiologic immunomodulation”), while at higher doses and particularly in its synthetic forms, such as prednisone and prednisolone, the hormone becomes immunosuppressive (“pharmacologic immunosuppression”) and produces additional adverse effects such as weight gain, truncal obesity, hypertension, glucose intolerance, and increased susceptibility to infections – the classic manifestations of hypercortisolemia seen in Cushing’s disease.41
Insufficiencies of cortisol production contribute to the development and perpetuation of allergies, fatigue, inflammation, and autoimmunity.42 The works of Drs John Tintera and William Jefferies have remained important despite being ignored by most endocrinologists; Jefferies’ books43 and articles are still widely available and provide concepts and clinically relevant applications. In a 1998 summary by Jefferies44 on the etiology of rheumatoid arthritis, he reviewed evidence suggesting that subacute hypoadrenalism results in insufficiencies of cortisol and DHEA, both of which are necessary for immunomodulation and immunocompetence; insufficiencies of these hormones leave the body vulnerable to chronic infections (ie, multifocal dysbiosis, as detailed previously) and the systemic proinflammatory sequelae that result in so-called “autoimmunity.”
Differential diagnoses in patients with low adrenal function include congenital adrenal hypoplasia, adrenoleukodystrophy, autoimmune Addison’s disease, and chronic hypopituitarism. A small, short-term, randomized, crossover clinical trial published in Lancet showed benefit of 5-10 mg/day of cortisol in patients with chronic fatigue syndrome.45 Supplementation with up to 20 mg per day of cortisol is physiologic; higher doses in the range of 40 mg per day may benefit patients, especially during times of stress, but are adrenosuppressive. My preference is to dose 10 mg first thing in the morning, then 5 mg in late morning, and 5 mg in mid-afternoon, as an attempt to replicate the diurnal variation and normal morning peak of cortisol levels. In patients with hypoadrenalism, administration of pregnenolone in doses of 10-60 mg in the morning may also be beneficial; DHEA supplementation is beneficial for patients with adrenal insufficiency.
Table 1. Diagnosis of Adrenal Insufficiency in Children and Adults
| Laboratory Finding | Interpretation |
| First-morning serum cortisol less than 8-10 µg/dL | Suggestive of adrenal insufficiency |
| Serum cortisol less than 18 µg/dL during illness or stress or with elevated ACTH | Highly suggestive of adrenal insufficiency |
| Serum cortisol less than 18 µg/dL 30-60 min following ACTH injection* | Diagnostic of adrenal insufficiency |
Serum cortisol that fails to double within 30-60 min following ACTH injection*This test involves 3 steps:
Cortisol production should double following ACTH injection |
Indicative of low adrenal reserve and impaired ability of the adrenal glands to respond to stress |
| Low output of adrenal hormones measured in 24-hr urine samples | (Provides sufficient objective data to justify a clinical trial of cortisol replacement in patients with a suggestive clinical picture and lack of contraindications) |
| *Proper dosing of cosyntropin/ACTH: Injections of “ACTH” are generally performed with the IV or IM injection of “cosyntropin” a synthetic peptide fragment of ACTH used for adrenal stimulation.46 According to a review by Wilson,47 the standard adult dose is 250 µg; suggested dosing for infants, younger children, and older children are 25 µg, 50 µg, and 100 µg, respectively.47 | |
DHEA (Insufficiency and Supraphysiologic Supplementation)
Patients with autoimmunity should be tested for DHEA insufficiency by measuring serum DHEA-sulfate. Insufficiencies should generally be corrected except in cases of concomitant hormone-responsive cancer, such as breast or prostate cancer. Physiologic doses are approximately 15-25 mg for women and 25-50 mg for men. However, many patients with autoimmunity will respond favorably to supraphysiologic doses in the range of 200 mg per day. The rationale for using high-dose DHEA in patients with autoimmune diseases is supported by the following:
- DHEA is a natural metabolite/hormone of the human body, made in the adrenal glands.
- Patients with autoimmune diseases are commonly treated with prednisone and other corticosteroids for months or years at a time. Use of prednisone causes adrenal suppression, with resultant suppression of DHEA levels.48
- As a consequence of prednisone treatment, many patients lose bone mass and develop osteoporosis. DHEA has been shown to reverse the osteoporosis and loss of bone mass induced by corticosteroid treatment.49
- DHEA shows no acute or subacute toxicity, even when used in supraphysiologic doses or used in sick patients. For example, in a study of 32 patients with HIV, DHEA doses of 750-2250 mg per day were well-tolerated and produced no dose-limiting adverse effects.50 This lack of toxicity compares favorably with any and all so-called “anti-rheumatic” drugs, nearly all of which show alarming comparable toxicity.
- DHEA is inexpensive. Even at the relatively high dose of 200 mg per day, the cost is less than $40 per month.
- When used at doses of 200 mg per day, DHEA safely provides clinical benefit for patients with various autoimmune diseases, including ulcerative colitis, Crohn’s disease,51 and lupus.52
- A DHEA dose of 200 mg per day allows lupus patients to reduce their dose of prednisone (thus avoiding its adverse effects) while also achieving symptomatic improvement. In other words, it allows for improved health and reduced medication use, and therefore fewer side effects.53
Based on these financial, safety, and effectiveness considerations, the administration of DHEA is thus reasonable for patients with moderate to severe autoimmune disease. Furthermore, since optimal clinical response appears to correlate with serum levels in the supraphysiologic range,54 treatment may be implemented with little regard for initial DHEA levels, particularly when 1) the dose of DHEA is kept as low as possible; 2) duration is kept as short as possible; 3) other interventions are used to address the underlying cause of the disease; and 4) the patient is deriving benefit and the risk-to-benefit ratio is favorable.
Final Word
Always use the lowest dose of any hormonal intervention necessary to achieve the desired result, based on dual consideration of laboratory results and the patient’s response to treatment. Lower doses of each intervention can be used when more interventions (eg, nutritional, mitochondrial, anti-dysbiotic) are used.
Footnote:
As of November 15, 2005, the word “orthoendocrinology” cannot be found either on PubMed/MEDLINE or Internet search using Google, Yahoo, or MSN search engines. I first published this term in Integrative Rheumatology in 2006, now published as Naturopathic Rheumatology v3.5.
 Alex Vasquez, DC, ND, DO, FACN, is the author of many books, including Functional Medicine Rheumatology, AntiViral Strategies and Immune Nutrition, and the recent 700-page textbook Functional Inflammology: Volume 1). He has also authored more than 100 publications in professional magazines and peer-reviewed medical journals, such as British Medical Journal (BMJ), Journal of Clinical Endocrinology and Metabolism, Journal of the American Medical Association (JAMA), Alternative Therapies in Health and Medicine, and Arthritis & Rheumatism, the official journal of the American College of Rheumatology.
Alex Vasquez, DC, ND, DO, FACN, is the author of many books, including Functional Medicine Rheumatology, AntiViral Strategies and Immune Nutrition, and the recent 700-page textbook Functional Inflammology: Volume 1). He has also authored more than 100 publications in professional magazines and peer-reviewed medical journals, such as British Medical Journal (BMJ), Journal of Clinical Endocrinology and Metabolism, Journal of the American Medical Association (JAMA), Alternative Therapies in Health and Medicine, and Arthritis & Rheumatism, the official journal of the American College of Rheumatology.
References:
- Vasquez A. Mitochondrial Nutrition and Endoplasmic Reticulum Stress in Primary Care. 2nd ed. CreateSpace Independent Publishing Platform; 2014.
- Bowman CA, Jeffcoate WJ, Pattrick M, Doherty M. Bilateral adhesive capsulitis, oligoarthritis and proximal myopathy as presentation of hypothyroidism. Br J Rheumatol. 1988;27(1):62-64.
- Vasquez A. Hypothyroidism and Hashimoto’s Disease in a Nutshell: New Perspectives for Doctors and Patients. CreateSpace Independent Publishing Platform; 2014.
- Vasquez A. Naturopathic Rheumatology, v3.5. International College of Human Nutrition and Functional Medicine; 2014.
- McMurray RW. Bromocriptine in rheumatic and autoimmune diseases. Semin Arthritis Rheum. 2001;31(1):21-32.
- Dostal C, Moszkorzova L, Musilova L, et al. Serum prolactin stress values in patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62(5):487-488.
- Moszkorzova L, Lacinova Z, Marek J, et al. Hyperprolactinaemia in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2002;20(6):807-812.
- Straub RH, Zeuner M, Lock G, et al. High prolactin and low dehydroepiandrosterone sulphate serum levels in patients with severe systemic sclerosis. Br J Rheumatol. 1997;36(4):426-432.
- La Montagna G, Baruffo A, Buono G, Valentini G. Dehydroepiandrosterone sulphate serum levels in systemic sclerosis. Clin Exp Rheumatol. 2001;19(1):21-26.
- Straub RH, Georgi J, Helmke K, et al. In polymyalgia rheumatica serum prolactin is positively correlated with the number of typical symptoms but not with typical inflammatory markers. Rheumatology (Oxford). 2002;41(4):423-429.
- Kramer CK, Tourinho TF, de Castro WP, da Costa Oliveira M. Association between systemic lupus erythematosus, rheumatoid arthritis, hyperprolactinemia and thyroid autoantibodies. Arch Med Res. 2005;36(1):54-58.
- Wuttke W, Jarry H, Christoffel V, et al. Chaste tree (Vitex agnus-castus)–pharmacology and clinical indications. Phytomedicine. 2003;10(4):348-357.
- Milewicz A, Gejdel E, Sworen H, et al. [Vitex agnus castus extract in the treatment of luteal phase defects due to latent hyperprolactinemia. Results of a randomized placebo-controlled double-blind study] Arzneimittelforschung. 1993;43(7):752-756.
- Meier B, Berger D, Hoberg E, et al. Pharmacological activities of Vitex agnus-castus extracts in vitro. Phytomedicine. 2000;7(5):373-381.
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
- Whitaker R. The case against antipsychotic drugs: a 50-year record of doing more harm than good. Med Hypotheses. 2004;62(1):5-13.
- Bone K. New Insights into Chaste Tree. Nutritional Wellness. November 2005. Available at: nutritionalwellness.com/archives/2005/nov/11_bone.php. Accessed November 1, 2014.
- Katzenschlager R, Evans A, Manson A, et al. Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75(12):1672-1677.
- Elwes RD, Crewes H, Chesterman LP, et al. Treatment of narcolepsy with L-tyrosine: double-blind placebo-controlled trial. Lancet. 1989;2(8671):1067-1069.
- Thomas JR, Lockwood PA, Singh A, Deuster PA. Tyrosine improves working memory in a multitasking environment. Pharmacol Biochem Behav. 1999;64(3):495-500.
- Deijen JB, Wientjes CJ, Vullinghs HF, et al. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res Bull. 1999;48(2):203-209.
- Waters WF, et al. A comparison of tyrosine against placebo, phentermine, caffeine, and D-amphetamine during sleep deprivation. Nutr Neurosci. 2003;6(4):221-35
- Ishizuka B, Quigley ME, Yen SS. Pituitary hormone release in response to food ingestion. J Clin Endocrinol Metab. 1983;57(6):1111-1116.
- Beers MH, Berkow R, eds. The Merck Manual of Diagnosis and Therapy. 17th ed Merck Research Laboratories; 1999:77-78.
- Alvarez-Nemegyei J, Cobarrubias-Cobos A, Escalante-Triay F, et al. Bromocriptine in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled study. Lupus. 1998;7(6):414-419.
- Serri O, Chik CL, Ur E, Ezzat S. Diagnosis and management of hyperprolactinemia. CMAJ. 2003;169(6):575-581.
- Sabuncu T, Arikan E, Tasan E, Hatemi H. Comparison of the effects of cabergoline and bromocriptine on prolactin levels in hyperprolactinemic patients. Intern Med. 2001;40(9):857-861.
- Verhelst J, Abs R, Maiter D, et al. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. 1999;84(7):2518-2522. Available at: jcem.endojournals.org/cgi/content/full/84/7/2518. Accessed October 30, 2005.
- Erb N, Pace AV, Delamere JP, Kitas JD. Control of unremitting rheumatoid arthritis by the prolactin antagonist cabergoline. Rheumatology (Oxford). 2001;40(2):237-239.
- Tanriverdi F, Silveira LF, MacColl GS, Bouloux PM. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J Endocrinol. 2003;176(3):293-304.
- Mayes MD. Epidemiologic studies of environmental agents and systemic autoimmune diseases. Environ Health Perspect. 1999;107 Suppl 5:743-748.
- Tengstrand B, Carlstrom K, Fellander-Tsai L, Hafstrom I. Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: high correlation between serum estradiol and current degree of inflammation. J Rheumatol. 2003;30(11):2338-2343.
- Jansson G. Oestrogen-induced enhancement of myeloperoxidase activity in human polymorphonuclear leukocytes–a possible cause of oxidative stress in inflammatory cells. Free Radic Res Commun. 1991;14(3):195-208.
- Bradlow HL, Telang NT, Sepkovic DW, Osborne MP. 2-hydroxyestrone: the ‘good’ estrogen. J Endocrinol. 1996;150 Suppl:S259-S265.
- McAlindon TE, Gulin J, Chen T, et al. Indole-3-carbinol in women with SLE: effect on estrogen metabolism and disease activity. Lupus. 2001;10(11):779-783.
- Life Extension Foundation. Male Hormone Restoration. LEF Web site. lef.org/protocols/prtcl-130c.shtml. Accessed October 30, 2005.
- Leder BZ, Rohrer JL, Rubin SD, et al. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab. 2004;89(3):1174-1180.
- Sereda D, Werth VP. Improvement in dermatomyositis rash associated with the use of antiestrogen medication. Arch Dermatol. 2006;142(1):70-72.
- Karagiannis A, Harsoulis F. Gonadal dysfunction in systemic diseases. Eur J Endocrinol. 2005;152(4):501-513.
- Booji A, Biewenga-Booji CM, Huber-Bruning O, et al. Androgens as adjuvant treatment in postmenopausal female patients with rheumatoid arthritis. Ann Rheum Dis. 1996;55(11):811-815.
- Kirk LF Jr, Hash RB, Katner HP, Jones T. Cushing’s disease: clinical manifestations and diagnostic evaluation. Am Fam Physician. 2000;62(5):1119-1127, 1133-1134.
- Jefferies WM. Mild adrenocortical deficiency, chronic allergies, autoimmune disorders and the chronic fatigue syndrome: a continuation of the cortisone story. Med Hypotheses. 1994;42(3):183-189.
- Jefferies W McK. Safe Uses of Cortisol. 2nd ed. Springfield, IL: Charles C Thomas Publisher; 1996.
- Jefferies WM. The etiology of rheumatoid arthritis. Med Hypotheses. 1998;51(2):111-114.
- Cleare AJ, Heap E, Malhi GS, et al. Low-dose hydrocortisone in chronic fatigue syndrome: a randomised crossover trial. Lancet. 1999;353(9151):455-458.
- Ogbru O. Cosyntropin (Cortrosyn). Reviewed August 15, 2014. Available at: http://www.medicinenet.com/cosyntropin-injectable/article.htm. Accessed December 7, 2006.
- Wilson TA. Adrenal Hypoplasia. Updated February 11, 2013. Medscape Web site. http://emedicine.medscape.com/article/918967-workup. Accessed November 18, 2005.
- Rittmaster RS, Givner ML. Effect of daily and alternate day low dose prednisone on serum cortisol and adrenal androgens in hirsute women. J Clin Endocrinol Metab. 1988;67(2):400-403.
- Mease PJ, Ginzler EM, Gluck OS, et al. Effects of prasterone on bone mineral density in women with systemic lupus erythematosus receiving chronic glucocorticoid therapy. J Rheumatol. 2005;32(4):616-621.
- Dyner TS, Lang W, Geaga J, et al. An open-label dose-escalation trial of oral dehydroepiandrosterone tolerance and pharmacokinetics in patients with HIV disease. J Acquir Immune Defic Syndr. 1993;6(5):459-465.
- Andus T, Klebl F, Rogler G, et al. Patients with refractory Crohn’s disease or ulcerative colitis respond to dehydroepiandrosterone: a pilot study. Aliment Pharmacol Ther. 2003;17(3):409-414.
- Chang DM, Lan JL, Lin HY, Luo SF. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(11):2924-2927.
- Petri MA, Lahita RG, Van Vollenhoven RF, et al. Effects of prasterone on corticosteroid requirements of women with systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2002;46(7):1820-1829.
- Barry NN, McGuire JL, van Vollenhoven RF. Dehydroepiandrosterone in systemic lupus erythematosus: relationship between dosage, serum levels, and clinical response. J Rheumatol. 1998;25(12):2352-2356.