Dugald Seely, ND
It will come as no surprise to most NDs that conventional oncologists often consider the addition of natural health products to the care of a cancer patient as potentially threatening to their model of care. Of course, there are the rare oncologists who welcome naturopathic interventions in the overall health of their patients and who may even have an implicit trust in our training and ability to treat cancer patients. Equally, however, if not with much greater frequency, are conventional oncologists who see any form of complementary intervention as dangerous “quackery” to be avoided at all costs. Fortunately, this position is losing favor; if not through education, then at least through patient demand.
The points for discussion that follow are meant for what we might consider the normative or standard conventional oncologist. As in most pursuits and professions, the majority of conventional oncologists are likely to be somewhat moderate and not take a position squarely within either of the extreme camps. This ‘normative oncologist’ may in fact be partially open to complementary care, albeit probably within a context of skepticism and ignorance. As a researcher and ND, I see any such skepticism as healthy and evidence of a desire and ability to distinguish what’s beneficial from what’s useless and, worse yet, from what’s potentially harmful. The skeptic is open to argument and, as such, can be convinced if the rationale is logical and reasonable. This article is meant to elucidate and address concerns that the skeptical normative oncologist might have before being open to encouraging or at least “allowing” their patients to proceed with the use of natural health products in conjunction with conventional treatment. This article is not meant to provide evidence to support any specific anticancer agents, but to provide the naturopathic oncologist with a strategy to address potential interactions between conventional treatment and complementary natural health product use.
Potential Interactions Between Chemotherapy and Natural Health Products
There are three ways in which the ingestion of natural health products can interact with chemotherapy: through effects that are physicochemical, pharmacokinetic and/or pharmacodynamic. Physicochemical interaction essentially refers to the possibility that the bioavailability of a compound is modified (usually reduced) through co-ingestion of a natural health product and drug compound. When co-ingestion of a natural health product and an oral chemotherapeutic agent occurs, there exists the possibility for this type of interaction. In the majority of cases, however, chemotherapy is provided by injection, making physicochemical interaction irrelevant. Increasing usage of molecularly targeted agents, often taken orally, however, may change the relative importance of this potential source of interaction. If this is of concern, then it is recommended that the patient not take any natural health products for at least 24 hours prior to ingestion of the orally prescribed chemotherapeutic or other anticancer agent.
Pharmokenetic Interactions: Drug Distribution and Concetration
When referring to pharmacokinetic interactions, the implication is that the time course distribution of a compound in the body undergoes alterations. From the perspective of a pharmacologist, this involves such variables as volume of distribution, body compartmentalization, half-life, excretion/clearance rates and metabolism. From the clinician’s perspective, however, what’s of most importance is that the concentration of a drug is not substantially pushed out of the therapeutic range. If pushed above the therapeutic range, the risk for toxicity increases; whereas if pushed below, the possibility for therapeutic failure correspondingly rises.
What is critical when considering pharmacokinetic interaction is the potential for changes to drug metabolism. The cytochrome P450 enzymes, primarily hepatic, are the most important enzymes responsible for drug metabolism and are most commonly assessed in drug interaction studies. So far, only St. John’s wort has demonstrated the ability to impact one of these important enzymes, CYP3A4, and may well adversely affect the pharmacokinetics and thus the ability of irinotecan to do its job (Mathijssen et al., 2002).
Evidence based on in vitro affinities of natural health products to specific CYP450 enzymes indicates the potential for clinically relevant interactions with chemotherapy (Venkataramanan et al., 2006). This issue is necessary to understand, as chemotherapeutic drugs come either as inherently active or as prodrugs that require in vivo metabolism to activate them. Toxicity is also directly mediated by the CYP450 enzyme system depending on whether these enzymes are involved in the metabolism, detoxification or elimination of chemotherapeutic agents. Metabolism of pharmaceuticals (active or prodrug) will invariably produce metabolites that also express various gradients of activity and toxicity.
 Pharmacodynamic Considerations: The Antioxidant Debate
Pharmacodynamic Considerations: The Antioxidant Debate
When referring to pharmacodynamic interactions, the implication is that there is an alteration in the biochemical or physiological mechanism of action of the drug. The concern is not that the concentration or distribution of the drug is changed, but that its ability to act effectively is attenuated. On the flip side, this area of interaction also allows for the possibility that synergies might be realized through co-administration.
The issue of concurrent administration of antioxidants to patients receiving chemotherapy is one that has generated a great deal of controversy (Block, 2004; D’Andrea, 2005; Conklin, 2000, 2004; Prasad, 2004; Beijnen & Schellens, 2004). There are valid concerns as to how antioxidants might interfere with the therapeutic efficacy of chemotherapy; however, the potential for some of these agents to offset any toxicity and side effects of chemotherapy is also a potent attractor.
Antioxidant depletion due to treatment with certain chemotherapies, such as platinum compounds, anthracyclines and alkylating agents, has clearly been established and is implicated in the development and severity of adverse effects. In a systematic review exploring the effect of antioxidant supplementation with conventional cancer therapy, it was observed that total antioxidant status drops during treatment (Ladas et al., 2004). In an observational study of children treated with combination chemotherapy for acute lymphoblastic leukemia (ALL), it was found that children with higher plasma antioxidants had fewer complications than those with lower antioxidant status (Kennedy et al., 2004).
Proponents of concurrent antioxidant use point out that human studies show that antioxidant supplements protect healthy cells from the toxic effects of chemotherapy drugs without also protecting cancer cells. On the other hand, those who argue against antioxidant use with chemotherapy are concerned that antioxidants will interfere with the cancer-killing effects of the cytotoxic chemotherapy drugs either directly or theoretically by inducing a natural health product/drug interaction (Kelly et al., 2000; Block & Gyllenhaal, 2002). Unfortunately, few randomized, controlled trials have investigated the effects of concomitant chemotherapy-antioxidant administration on survival. Patients are left with a difficult dilemma: Should they take antioxidants to help protect healthy cells against chemotherapy’s proven toxicity, or should they avoid all antioxidant supplementation because of the possibility that they will attenuate the cancer-killing effects of chemotherapy?
To further complicate matters, certain natural health products may augment the efficacy of some chemotherapy agents. An example of this includes work done on whey protein and other cysteine precursors that modulate glutathione levels (Wang et al., 1996; Baruchel et al., 1995). Glutathione, a potent antioxidant, appears to be concentrated in cancer cells and is one of the mechanisms by which cancer cells acquire drug resistance to chemotherapy. Interestingly, ingestion of whey protein causes depletion of glutathione in cancer cells (chemosensitization), while at the same time increasing glutathione production in healthy cells (chemoprotection). Therefore, the action of whey protein or cysteine precursors may be doubly beneficial (Wang et al., 1996; Baruchel et al., 1995; Bounous, 2000; Panetta et al., 2002; Baruchel & Viau, 1996). Chemotherapy drugs (particularly the alkylating agents, anthracyclines, platinum compounds and topoisomerase II inhibitors) often exert their therapeutic (and toxic) effects by forming reactive oxygen species and free radicals. In theory, it is thought that antioxidants may reduce the efficacy of anticancer agents by quenching free radicals, but there are several examples of adjunctive agents – such as mesna (Links and Lewis, 1999) and amifostine (Fouladi et al., 2001), whose protective effects are attributed to the quenching of free radicals – that do not appear to decrease the efficacy of chemotherapy. Supplementation of individuals receiving chemotherapy with antioxidants may even be viewed as similar to leucovorin rescue of patients being treated with high-dose methotrexate. This derivative of folic acid is usually given 24 hours after methotrexate, as most of the complications and side effects of methotrexate can be either prevented or treated by using leucovorin.
 Pharmacodynamic Considerations: Modulation of Cancer Cell Chemosensitivity
Pharmacodynamic Considerations: Modulation of Cancer Cell Chemosensitivity
In addition to the potential impact natural health products may have on chemotherapeutic effect on cancer cells and normal cells, there also exists the potential for the natural health products to modulate cellular resistance to chemotherapy. Synergy between a natural health product and chemotherapy could lead to enhanced cancer cell cytotoxicity by reducing cellular chemoresistance. The cellular agent most often implicated in this process is P-glycoprotein, a transmembrane efflux transporter (Kim, 2002). P-glycoproteins play a large role in the distribution and elimination of many clinically important therapeutic substances.
Strategy to Control Potential Interactions
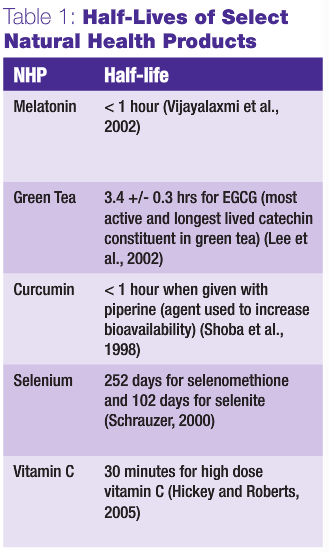
In principle, it takes five half-lives to reach steady state and five half-lives to eliminate virtually all of a drug from the body. Knowing the half-lives of both natural health products and chemotherapy can allow for design of a dosing regime that will theoretically ensure that both agents are not present in the blood stream at the same time. In Figure 1, three possible scenarios of natural health product-drug combinations are depicted. In Figure1a, the likelihood of interaction is strongly reduced, as administration of the natural health product is stopped and sufficient time is allowed for it to wash out before exposure to intravenously administered chemotherapy. The same applies post-treatment with an adequate wash-out period for the chemotherapy drug before natural health product ingestion resumes. In Figure 1b, the natural health product is given right up to and immediately following chemotherapy, thereby greatly increasing the risk of interaction. Finally, in Figure 1c, the greatest risk for interaction is depicted should natural health product ingestion not be stopped during chemotherapy administration.
In the case where we cannot predict whether or not an interaction is likely to occur, many clinicians, both naturopathic and conventional, might argue that the prudent choice is to ensure that the first situation (Figure 1a) is maintained. Chemotherapy is highly toxic, with a narrow therapeutic window, and it is important that its efficacy and toxicity profile not be inappropriately modified. In the case where an assumed benefit occurs through concurrent use of a natural health product and chemotherapy, then the third situation, Figure 1c, may in fact be desired, with no delay advised between the natural health product and chemotherapy. This situation is the goal – where a natural health product is able to reduce toxicity to normal tissue without reducing the chemotherapy’s efficacy. The other scenario where this would be desirable is in the event that the natural health product is known to synergize with the chemotherapy, thereby increasing its potency. Unfortunately, however, as yet there does not appear to be any combination of natural health products and chemotherapy that we can say with certainty will lead to clinical benefit and not to harm.
 The window for pharmacokinetic interaction is also modified by the length of time the metabolizing enzymes (predominantly the CYP450 enzymes) may be induced or inhibited. Inhibition of drug metabolizing enzymes can be classified as reversible, quasi-irreversible or irreversible. Most often, drug interactions result due to competitive reversible inhibition (Alfaro and Piscitelli, 2001). When this situation arises, we can expect that inhibition will last only as long as the inhibitor is present. Thus, the half-life is a reliable estimator of the potential window of interaction, and inhibition should not last longer than the time required for substrate (natural health product) clearance. In the case where metabolizing enzymes are down-regulated by irreversible inhibition, the window of interaction may expand beyond the range of five half-lives. Thus, a more conservative approach is required to establish a safe time frame for natural health product avoidance prior to commencing chemotherapy.
The window for pharmacokinetic interaction is also modified by the length of time the metabolizing enzymes (predominantly the CYP450 enzymes) may be induced or inhibited. Inhibition of drug metabolizing enzymes can be classified as reversible, quasi-irreversible or irreversible. Most often, drug interactions result due to competitive reversible inhibition (Alfaro and Piscitelli, 2001). When this situation arises, we can expect that inhibition will last only as long as the inhibitor is present. Thus, the half-life is a reliable estimator of the potential window of interaction, and inhibition should not last longer than the time required for substrate (natural health product) clearance. In the case where metabolizing enzymes are down-regulated by irreversible inhibition, the window of interaction may expand beyond the range of five half-lives. Thus, a more conservative approach is required to establish a safe time frame for natural health product avoidance prior to commencing chemotherapy.
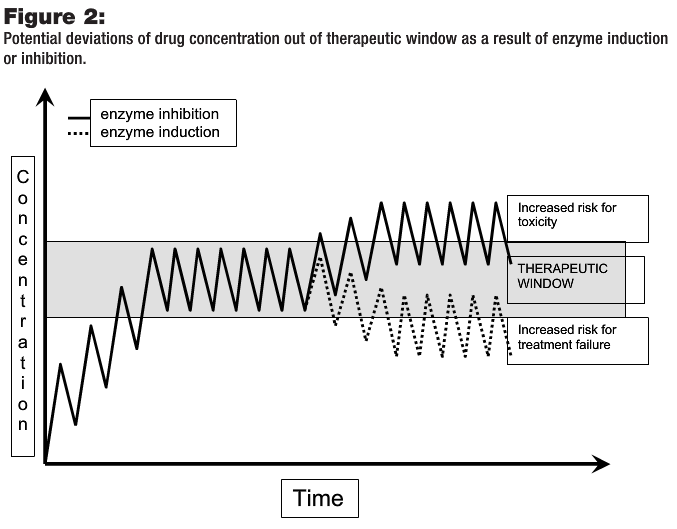
In the case of induction, changes in enzyme expression result from increased DNA transcription and synthesis of CYP450 enzymes. The time course of induction also depends on the elimination half-life of the inducer as well as the time required for enzyme degradation and new enzyme production (Alfaro and Piscitelli, 2001). As opposed to competitive enzyme inhibition, however, CYP450 induction will likely have a carryover, or “hangover,” effect. Thus, a longer delay prior to chemotherapy may be a wiser course if enzyme induction is expected. Figure 2 depicts drug concentration changes in the presence of inducers or inhibitors. In the case of chemotherapy drugs with narrow therapeutic windows, the potential for toxicity or therapeutic failure is especially high.
When to Stop Natural Health Products’ Consumption Prior to Chemotherapy
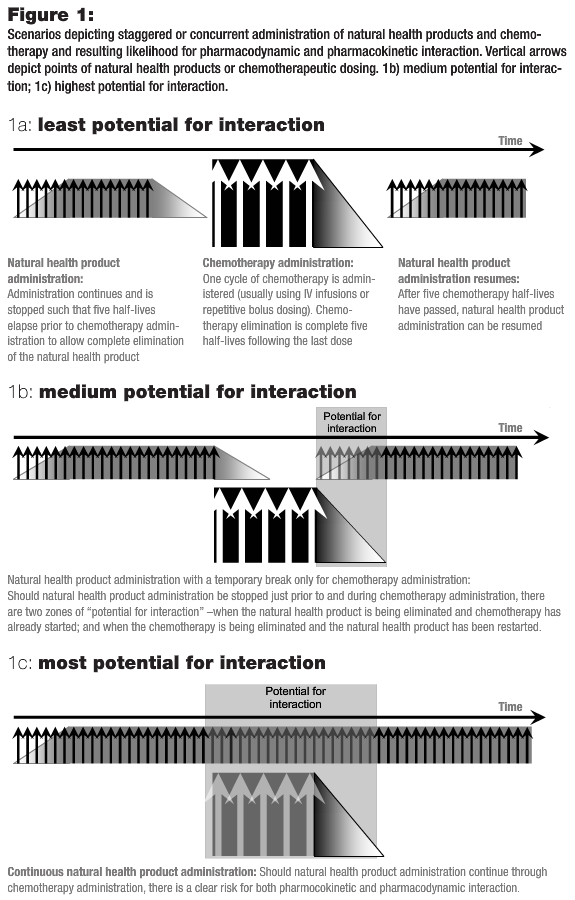
The evidence regarding natural health products’ effect on metabolizing enzymes is lacking not only with regards to the direction (i.e., inhibition or induction), but the mechanism of interaction is even more poorly characterized. Consequently, pharmacokinetic interactions between natural health products and chemotherapy are virtually impossible to reliably predict. Table 1 details the half-lives of a selection of natural health products. It is apparent that each of them (with the exception of selenium) has a half-life that is less than four hours. Thus, one day free from these natural health products will have exceeded the five half-life limit and should be adequate for full elimination, thereby avoiding any potential interaction as depicted in Figure 1a. In addition, there is no evidence that these natural health products affect the CYP3A4 or CYP2D6 isozymes, and thus are less likely to alter the metabolism of most chemotherapy drugs (Seely et al., 2007).
When no information is available as to the half-life of the natural health product or on the inductive or inhibitory effect on drug metabolism, greater caution is warranted. Stopping the natural health product one week prior to chemotherapy is probably adequate, but again must be moderated by patient/family wishes and the comfort level of yourself and the medical oncologist. In the situation where clear evidence of interaction is available, a situation applicable to St. John’s wort, a strict recommendation to avoid consumption of this product prior to therapy is probably the best choice. Unfortunately, with respect to clear evidence of interaction, St. John’s wort is the exception. It appears likely that the risk of pharmacokinetic interaction between most natural health products and chemotherapy is overblown; however, as yet we cannot be certain of this.
When to Restart Natural Health Product Consumption Post Chemotherapy
Advice as to when a patient should restart a natural health product after chemotherapy should deal in particular with the pharmacokinetic profile of the chemotherapeutic drug. Typically, the half-lives for commonly used chemotherapeutic agents range anywhere between 1 to 30 hours (Seely et al., 2007). Typical chemotherapeutic dosing follows a schedule wherein high doses of chemotherapy are given sequentially, followed by a two- to three-week rest period. As the therapeutic window is mostly maintained during this sequential dosing, if natural health product interaction is uncertain, consumption during this period should be avoided. Once the chemotherapy is stopped, however, and three half-lives have elapsed, the therapeutic window will be closed. Based on this rational approach, it is at this time that natural health product use can be safely reintroduced.
As the evidence accumulates and we become more aware of how different natural health products interact with conventional therapies, we will be better able to make predictions and prescribe more effectively. A specific area wherein the greatest potential exists to improve health includes the reduction of side effects caused by chemotherapy. Even more exciting, however speculative, is the possibility of arresting cancer progression and potentially even the induction of remission by the use of cocktails of natural health products wherein synergies take place to specifically target physiological weak points of cancer. Perhaps planned interactions (i.e., synergies) will be especially beneficial through the addition of natural health products to new molecular therapies as exemplified by bevacizumab or trastuzumab. It is imperative that basic and clinical research explore these avenues so naturopathic and conventional oncologists can be better equipped to deal with this complex and devastating disease.

Dugald Seely, ND has a small oncology-based practice. The majority of his time, however, is dedicated to the research of therapies used by NDs, with a primary focus in the area of cancer research. Dr. Seely is director of research at CCNM and is active in building the internal research capacity of the college as well as being the primary investigator on a 5-year study assessing the effectiveness of melatonin in non-small-cell lung cancer. Dr. Seely is also active in the development and dissemination of evidence regarding the effectiveness of naturopathic medicine, with publications in more than 20 peer-reviewed articles, including three book chapters.
References
Mathijssen RH et al: Effects of St. John’s wort on irinotecan metabolism, J Natl Cancer Inst 94:1247-1249, 2002.
Venkataramanan R et al: In vitro and in vivo assessment of herb drug interactions, Life Sci 78:2105-2115, 2006.
Block KI: Antioxidants and cancer therapy: furthering the debate, Integr Cancer Ther 3:342-348, 2004.
D’Andrea GM: Use of antioxidants during chemotherapy and radiotherapy should be avoided, CA Cancer J Clin 55:319-321, 2005.
Conklin KA: Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects, Nutr Cancer 37:1-18, 2000.
Conklin KA: Cancer chemotherapy and antioxidants, J Nutr 134:3201S-3204S, 2004.
Prasad KN. Multiple dietary antioxidants enhance the efficacy of standard and experimental cancer therapies and decrease their toxicity, Integr Cancer Ther 3:310-322, 2004.
Beijnen JH, Schellens JH: Drug interactions in oncology, Lancet Oncol 5:489-496, 2004.
Ladas EJ et al: Antioxidants and cancer therapy: a systematic review, J Clin Oncol 22:517-528, 2004.
Kennedy DD et al: Low antioxidant vitamin intakes are associated with increases in adverse effects of chemotherapy in children with acute lymphoblastic leukemia, Am J Clin Nutr 79:1029-1036, 2004.
Kelly KM et al: Use of unconventional therapies by children with cancer at an urban medical center, J Pediatr Hematol Oncol 22:412-416, 2000.
Block KI, Gyllenhaal C: Clinical corner: herb-drug interactions in cancer chemotherapy: theoretical concerns regarding drug metabolizing enzymes, Integr Cancer Ther 1:83-89, 2002.
Wang T et al: Modulation of glutathione by a cysteine pro-drug enhances in vivo tumor response, J Pharmacol Exp Ther 276:1169-1173, 1996.
Baruchel S et al: In vivo selective modulation of tissue glutathione in a rat mammary carcinoma model, Biochem Pharmacol 50:1505-1508, 1995.
Bounous G: Whey protein concentrate (WPC) and glutathione modulation in cancer treatment, Anticancer Res 20:4785-4792, 2000.
Panetta JC et al: Limited and optimal sampling strategies for etoposide and etoposide catechol in children with leukemia, J Pharmacokinet Pharmacodyn 29:171-188, 2002.
Baruchel S, Viau G: In vitro selective modulation of cellular glutathione by a humanized native milk protein isolate in normal cells and rat mammary carcinoma model, Anticancer Res 16:1095-1099, 1996.
Links M, Lewis C: Chemoprotectants: a review of their clinical pharmacology and therapeutic efficacy, Drugs 57:293-308, 1999.
Fouladi M et al: Phase I trial of a twice-daily regimen of amifostine with ifosfamide, carboplatin, and etoposide chemotherapy in children with refractory carcinoma, Cancer 92:914-923, 2001.
Kim RB: Drugs as P-glycoprotein substrates, inhibitors, and inducers, Drug Metab Rev 34:47-54, 2002.
Alfaro CL, Piscitelli SC: Drug interactions. In Atkinson AJ et al: Principals of Clinical Pharmacology, San Diego, 2001, Academic Press, pp 167-180.
Seely D et al: A strategy for controlling potential interactions between natural health products and chemotherapy: a review in pediatric oncology, J Pediatr Hematol Oncol 29:32-47, 2007.
Vijayalaxmi, Thomas CR et al: Melatonin: from basic research to cancer treatment clinics, J Clin Oncol 20:2575-2601, 2002.
Lee MJ et al: Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability, Cancer Epidemiol Biomarkers Prev 11:1025-1032, 2002.
Shoba G et al: Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers, Planta Med 64:353-356, 1998.
Schrauzer GN: Selenomethionine: a review of its nutritional significance, metabolism and toxicity, J Nutr 130:1653-1656, 2000.
Hickey S, Roberts H: Misleading information on the properties of vitamin C, PLoS Med 2:e307; author reply e309, 2005.





