Node Smith, ND,
The first time Lori Tipton tried MDMA, she was skeptical it would make a difference. “I really was, at the beginning, very nervous,” Tipton said.
MDMA for PTSD
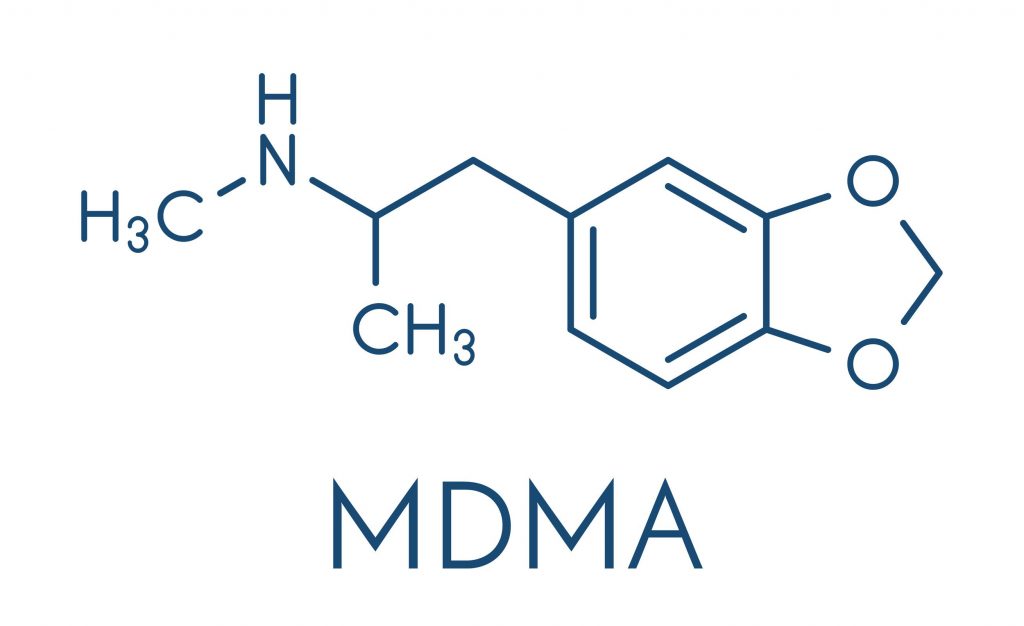
MDMA is the main ingredient in the club drug known as ecstasy or molly. But Tipton wasn’t taking pills sold on the street to get high. She was trying to treat her post-traumatic stress disorder by participating in a clinical trial.
After taking a dose of pure MDMA, Tipton lay in a quiet room with two specially trained psychotherapists. They sat next to her as she recalled some of her deepest traumas, such as discovering her mother’s body after Tipton’s mother killed two people and then herself in a murder-suicide.
“In the embrace of MDMA,” Tipton said, she could revisit that moment without the usual terror and panic. “I was able to find such empathy for myself.”
Scientists are testing how pharmaceutical-grade MDMA can be used in combination with psychotherapy to help patients with a severe form of PTSD that haven’t responded to other treatments. Unlike street drugs, which may be adulterated and unsafe, researchers use a pure, precisely dosed form of the drug.
MDMA not yet available as treatment for PTSD outside clinical trials
MDMA is not yet available as a treatment for PTSD outside of clinical trials. But proponents are aiming for approval by the Food and Drug Administration, which granted breakthrough therapy status to MDMA-assisted psychotherapy in 2017.
Researchers are conducting Phase 3 clinical trials at more than a dozen sites. Clinicians who treat PTSD are hopeful the next round of trials will show that MDMA treatment is an effective option to relieve patient suffering.
“The problem is we haven’t had a new drug to treat PTSD in over 17 years,” said Dr. Sue Sisley, a physician and president of the Scottsdale Research Institute, based in Arizona. “There are certain illnesses that are just intractable and not responsive to traditional therapy, and we need to start thinking more broadly.”
But MDMA is a Schedule I controlled substance, which means it currently has no accepted medical use and has a “high potential for abuse” (something that MDMA’s therapeutic proponents dispute). Because of that designation, the current research trials are privately funded by the Multidisciplinary Association for Psychedelic Studies, or MAPS.
Struggling for years with PTSD
Tipton struggled for years with PTSD before she was treated with MDMA. She said life with PTSD was like “seeing the world through dirty goggles.”
“Anywhere I would feel unsafe,” the 40-year-old from New Orleans said. “I would feel like I had to always be vigilant because if I didn’t, something bad was going to happen.”
Tipton described her 20s as a catalog of tragedy and trauma. It began when her brother fatally overdosed in her home. After his death, she began caring for her mother, who struggled with mental illness. In 2005, Tipton’s mother killed two people and then herself. Tipton discovered their bodies.
“I completely just disassociated. I couldn’t believe what I was seeing,” Tipton said.
The traumas continued to pile up. The place she lived was destroyed when Hurricane Katrina hit New Orleans, and the following year, she was raped.
Phase 2 clinical trials for MDMA-assisted psychotherapy
As the years went by, Tipton had panic attacks and terrible anxiety. She tried everything to treat her symptoms: talk therapy, antidepressants, hypnotherapy, meditation and yoga. Nothing worked. She went through life exhausted and apathetic, constantly triggered and struggling to be intimate with people close to her.
Then Tipton enrolled in the Phase 2 clinical trials for MDMA-assisted psychotherapy.
MDMA and Therapy Together
MDMA was first synthesized in 1912, and its therapeutic benefits were studied in the 1970s. But those efforts stalled when the U.S. federal government — in light of the growing popularity of ecstasy as a recreational drug — designated it a Schedule I drug in 1985.
In recent years, research has resumed, funded by private sponsors such as MAPS.
The treatment protocol
The treatment protocol in the current trial calls for a 12-week course of psychotherapy with specially trained therapists. During that time, there are two or three daylong sessions, which begin with the patient taking a calibrated dose of MDMA in pill form.
A team of two therapists, generally one man and one woman, then guide the patient through the eight-hour MDMA “session.” Later, there’s follow-up talk therapy, without the drug, to help the patient process feelings, thoughts or impressions that came up while under the influence of the drug.
“MDMA allows you to contact feelings and sensations in a much more direct way,” said Saj Razvi, a Colorado-based psychotherapist who was a clinical investigator in the Phase 2 trials.
How MDMA works in the brain is not completely understood. The psychoactive drug boosts chemicals like serotonin and oxytocin. It also tamps down activity in the amygdala, a part of the brain that processes fear. This may lead to a state characterized by heightened feelings of safety and social connection.
“Trauma happens in isolation,” Razvi said. “One of the things that MDMA does is, really, lets you know that you are not alone.”
PTSD In Remission
After the Phase 2 trials of MDMA-assisted treatments concluded in 2017, researchers found that 54% of the 72 patients who took MDMA had improved to the point that they no longer fit the diagnosis for PTSD (compared with 23% in the control group).
And the beneficial effects of the treatment appeared to increase over time. A year later, the number who no longer had PTSD had risen to 68%.
“That was astonishing,” Sisley said. “Even with the best pharmaceutical regimen, you rarely ever see patients go into remission.”
She said she hopes to offer her patients MDMA-assisted psychotherapy as soon as possible, maybe before the drug receives full FDA approval.
Brad Burge, a spokesman for MAPS, said that, beyond sponsoring the MDMA trial, the organization is working to get the FDA to include the drug in its expanded access program, which can allow individual patients to be approved to use drugs that are still being studied.
Burge said the goal is to make MDMA-assisted psychotherapy available as a prescription treatment in a specialty clinic to anyone with PTSD.
And MAPS is working to persuade public and private insurance plans to cover the treatment, Burge said. He estimates that for patients paying entirely out-of-pocket, a 12-week course of treatment would cost between $5,000 and $10,000.
Most of the cost is for the guided therapy, not the actual drug.
Transformative Treatment
Tipton describes her treatment with MDMA as transformative. She was able to let go of the troubling feelings surrounding her mother’s death. And, she unearthed other memories, too, feelings of joy that had been sealed away.
By her last MDMA session, Tipton was even able to talk about her sexual assault.
A year later, she was reassessed and no longer qualified as having PTSD. Tipton said she believes the treatment saved her life.
“Everything is at my fingertips for me in a way that it never was before,” she said. “I want that for everybody.”
|
This article was reprinted from khn.org with permission from the Henry J. Kaiser Family Foundation. Kaiser Health News, an editorially independent news service, is a program of the Kaiser Family Foundation, a nonpartisan health care policy research organization unaffiliated with Kaiser Permanente. |
 Node Smith, ND, is a naturopathic physician in Humboldt, Saskatchewan and associate editor and continuing education director for NDNR. His mission is serving relationships that support the process of transformation, and that ultimately lead to healthier people, businesses and communities. His primary therapeutic tools include counselling, homeopathy, diet and the use of cold water combined with exercise. Node considers health to be a reflection of the relationships a person or a business has with themselves, with God and with those around them. In order to cure disease and to heal, these relationships must be specifically considered. Node has worked intimately with many groups and organizations within the naturopathic profession, and helped found the non-profit, Association for Naturopathic Revitalization (ANR), which works to promote and facilitate experiential education in vitalism.
Node Smith, ND, is a naturopathic physician in Humboldt, Saskatchewan and associate editor and continuing education director for NDNR. His mission is serving relationships that support the process of transformation, and that ultimately lead to healthier people, businesses and communities. His primary therapeutic tools include counselling, homeopathy, diet and the use of cold water combined with exercise. Node considers health to be a reflection of the relationships a person or a business has with themselves, with God and with those around them. In order to cure disease and to heal, these relationships must be specifically considered. Node has worked intimately with many groups and organizations within the naturopathic profession, and helped found the non-profit, Association for Naturopathic Revitalization (ANR), which works to promote and facilitate experiential education in vitalism.
Node Smith graduated from the National University of Natural Medicine (NUNM) in 2017, and is currently licensed as a naturopathic physician in Oregon and working towards becoming licensed in Saskatchewan, Canada as well.