Part Two—Prevention and Treatment
Pamela Hutchison, BSc, ND
With the proportion of the population older than 65 years in rapid ascent, the burden on the Canadian and American healthcare systems due to age-related cognitive disorders will be immense. Conventional medical treatment focuses on medication, which at best limits and delays decline because dementia once it is apparent represents the cumulative effects of decades of degenerative neurological damage. Inflammation has a major role in this process, and although much damage has been done by the time a person is in his or her 60s, evidence shows that when caught early we can effect change in these conditions. Addressing inflammation head on and early with lifestyle, nutritional, and supplemental interventions can prevent, limit, treat, or augment conventional treatment, improving outcomes for the patient and, if adopted on a large scale, for the medical system as a whole.
Treating Temporary and Correctable Causes of Cognitive Decline
Initial assessment focuses on identifying patients at risk of cognitive decline and those in various stages of impairment. A crucial element to the assessment of this population is a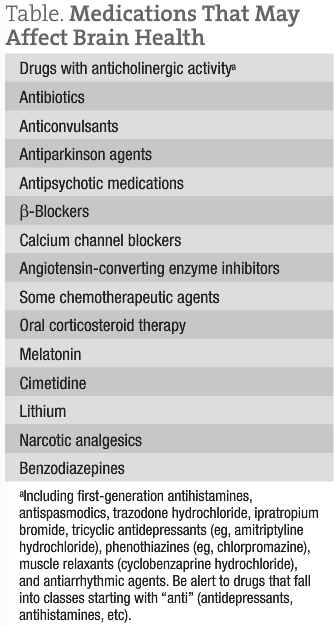 ddressing any causes of temporary cognitive decline and treating issues that would exacerbate any underlying pathologic condition.
ddressing any causes of temporary cognitive decline and treating issues that would exacerbate any underlying pathologic condition.
The most common conditions that will limit cognitive function in older populations are the following: hypotension, low iron level, low vitamin B12 level, hypothyroidism, uncontrolled seizure disorders, sleep disorders, and depression.
Patients with these concerns will often see improvements in their cognitive capacity with correction of the disorder. Repetition of functional assessments after addressing these concerns is advisable.
Often, these patients will be taking multiple medications, many of which have the capacity to affect brain health. The Table lists medications to be on the lookout for.
Many of these drugs cause cognitive impairment in a dose-related fashion. Dose reductions and medication changes or replacements should be managed to preserve the original therapeutic benefit for the patient.
Diet
The Mediterranean Diet
The Mediterranean diet (MeDi) is the most studied and validated diet for prevention of all-cause dementia. The diet is the confluence of high consumption of fruits, vegetables, nuts, seeds, legumes, beans, whole grains, and olive oil; frequent consumption of fish and seafood; moderate consumption of red wine, poultry, eggs, cheese, and yogurt; and rare consumption of red meats, organ meats, butter, and sugar.
In a prospective cohort study1 of 2258 individuals over 1½ years, those who scored in the highest tertile on a 9-point scale for adherence to the MeDi had significantly reduced risk of Alzheimer dementia (AD) compared with those who scored in the lowest tertile. Another study2 of 2148 individuals 65 years and older found that a dietary pattern of high intake of salad dressings, nuts, fish, tomatoes, poultry, cruciferous vegetables, fruits, and dark and green leafy vegetables and lower intake of high-fat dairy products, red meat, organ meats, and butter was associated with lower risk of AD. Mild cognitive impairment may also be prevented with adherence to the MeDi. Among a group of 1233 individuals aged 70 to 89 years, incidence of mild cognitive impairment was less with stronger adherence to the MeDi.3 In general, this diet is beneficial to cognitive function on the whole, but the mechanism or mechanisms have yet to be fully understood. Biomarkers for inflammation (high-sensitivity C-reactive protein) are lower in those who adhere to the MeDi, but those indicating dysglycemia (fasting insulin and adiponectin) do not correlate with adherence to the diet.4
It is a safe assumption that most patients regardless of their test outcomes would benefit from this diet. As NDs, we may consider further dietary interventions to limit inflammatory activity by testing for food allergies and sensitivities and removing offending foods, particularly in patients with elevated levels of high-sensitivity C-reactive protein and interleukin 6 (IL-6). However, evidence for this treatment strategy was not found in the literature to date.
Green Tea Polyphenols
The main flavonoid of interest in green tea is the catechin known as ECGC (epigallocatechin gallate), which has many favorable biological actions in the brain, including iron and copper chelation,5 free radical scavenging, and direct anti-inflammatory activity.6 Green tea catechins also seem to affect neuronal mitochondrial function, cell signal transduction pathways, and cell survival and death genes in ways that confer neuroprotection and promote neuronal recovery.7
Although promising, human data on the effect of green tea in the brain are limited, as is epidemiological evidence. A recent double-blind placebo-controlled trial of a supplement combining green tea extract and l-theanine showed mild improvements in memory and attention tasks after 16 weeks compared with placebo; electroencephalographic testing after a single dose of the supplement showed increased theta-wave activity (a marker of alertness) in temporal, frontal, parietal, and occipital lobes.8 A Chinese cross-sectional study9 of 716 adults 55 years and older found that total tea consumption (green and black) was independently associated with better performance on neuropsychological tests for global cognition, memory, executive function, and information processing speed.
The consumption of 2 to 3 cups of green tea per day is advisable in general for prevention of cognitive decline. Treatment with green tea has yet to be well studied, but early evidence indicates that mild treatment effects may be possible with concentrated supplements.
Supplementation
Fish Oils
Omega-3 fatty acids are major players in brain health in general. The docosahexaenoic acid (DHA) fraction in particular seems to be preventive of AD through various mechanisms, including limiting the production of the β-amyloid peptide (an initiating factor in AD), moderating factors implicated in neurofibrillary tangle development, reducing neuroinflammation, and increasing brain levels of brain-derived neurotrophic factor (a neuroprotective compound).10-12 Clinical trials using DHA or fish oil alone demonstrate a capacity to slow early stages of progression, but DHA supplementation at 2 g/d over 18 months showed no appreciable effects on cognitive function or AD-associated brain atrophy in individuals with mild to moderate AD.13
Supplementation with fish oils or DHA seems to be of greatest benefit in preventing AD or slowing early stages of progression.
Colostrinin
Colostrinin is a proline-rich mixture of polypeptides isolated from colostrums. In vitro evidence has shown that it reduces β-amyloid–induced cytotoxicity and elevates the antioxidant superoxide dismutase 1 in neuroblastoma cell lines.14 In animal investigations, it moderated cytokine tumor necrosis factor, IL-6, IL-10, and interferon induction and prevented the formation of β-amyloid aggregates, while reducing those already formed.15 A placebo-controlled study16 of patients with AD taking 100 mg of colostrinin (every other day for 3 weeks, followed by a 2-week hiatus) over 16 months demonstrated statistically significant improvement in cognitive performance on neurological tests by the treatment group. A subsequent multicenter double-blind placebo-controlled trial among patients with AD had similar outcomes over a shorter period of 15 weeks; patients with mild AD symptoms showed the most impressive response to colostrinin therapy.17
Colostrinin is a promising treatment that calms the immune system and reduces inflammation in the central nervous system. The typical dosing pattern is 100 mg every other day for 3 weeks, followed by a 2-week break. This is done to prevent oversedation of the immune system. Given its effect on immunity—and because of a lack of large high-powered trials to demonstrate safety with long-term use—colostrinin should be reserved for treatment of true cognitive impairment and AD and should not be used for prevention. At this time, there is no evidence of benefit for patients with vascular dementia.
Vitamin B12 and Folic Acid
Dementia is one of the many symptoms of vitamin B12 deficiency. Older persons are at higher risk of vitamin B12 deficiency because of increased incidence of atrophic gastritis, hypochlorohydria, and antacid use. Even subclinical deficiencies can cause memory loss and confusion in older patients. Vitamin B12 supplementation beyond physiological levels to treat AD or cognitive decline has insufficient support from the literature. Few double-blind placebo-controlled trials have been performed, and they have not shown any effect beyond placebo in treatment of AD,18 a surprising finding given that a low vitamin B12 level is strongly correlated with increased risk of poor cognitive function, cognitive decline, and AD. To date, no randomized trials have investigated vitamin B12 supplementation to treat mild cognitive impairment, early cognitive decline, or vascular dementia, nor have any assessed vitamin B12 injections for any form of cognitive decline.
Low folate levels, along with low vitamin B12 level and high homocysteine level, are associated with an increased risk of cognitive impairment. Folate supplementation has been studied as a preventive treatment for AD, as well as an adjunctive therapy to traditional cholinesterase inhibitors in AD therapy. Supplementation of 800 mcg/d of folic acid over 3 years in healthy older adults with high homocysteine levels resulted in significant improvement in global functioning, memory storage, and processing speed.19 Patients taking cholinesterase inhibitors for treatment of AD who were given 1 mg/d of folic acid demonstrated significant improvement on rating scales for activities of daily living and social behavior.19
All patients reporting memory loss or cognitive impairment or scoring poorly on the Mini-Mental State Examination should be tested for vitamin B12 level. Replenishment to optimal levels is recommended and may improve or repair cognitive function if the deficiency was the cause of the impairment. Folate levels should also be tested to rule out deficiency. There is evidence that folic acid supplementation may assist in prevention and treatment of mild cognitive impairment, particularly in individuals with elevated homocysteine levels. Clinical judgment should be exercised in advising the use of these vitamins for patients having cognitive impairment or for those wishing to prevent it, keeping in mind that they are extremely safe when given together but that few studies in the literature provide favorable epidemiological data.
Other Considerations
A growing body of research exists on prevention and treatment of age-related cognitive decline. There are varying levels of evidence for the use of melatonin, S-adenosylmethionine, ginkgo, l-theanine, curcumin, phosphatidyl choline, ginger, a high-nitrate diet, and selenium for treatment or prevention.
Exercise on its own and in conjunction with various therapies has shown positive effects on cognition.20 Improving sleep time and quality will also improve brain health. For patients with vascular dementia, lowering blood pressure is essential; however, avoidance of hypotensive events in these individuals is also necessary, so targets for blood pressure should remain modest. Blood pressures of 135/85 mm Hg are sufficient control in this population.
Age-related cognitive decline develops over decades, so patients should be encouraged to allow time for natural interventions to take effect. A staged approach of thorough assessment and targeted treatment or prevention strategies will bear fruit for many patients, and follow-up physiological and functional testing will keep patients motivated to maintain their treatment plans.
Dr. Pamela Hutchison, BSc, ND, has practiced with a clinical focus on mental health, neurology, and neurobehavioral disorders in adults and children for more than 10 years. She is clinic director of Acacia Integrative Health Clinic in Victoria, British Columbia, Canada, a multidisciplinary clinic with a team approach to healthcare.
References
1. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912-921.
2. Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol. 2010;67(6):699-706.
3. Roberts RO, Geda YE, Cerhan JR, et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29(5):413-423.
4. Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):483-492.
5. Mandel S, Amit T, Bar-Am O, Youdim MB. Iron dysregulation in Alzheimer’s disease: multimodal brain permeable iron chelating drugs, possessing neuroprotective-neurorescue and amyloid precursor protein-processing regulatory activities as therapeutic agents. Prog Neurobiol. 2007;82(6):348-360.
6. Ramesh BN, Rao TS, Prakasam A, Sambamurti K, Rao KS. Neuronutrition and Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1123-1139.
7. Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MB. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J Nutr. 2008;138(8):1578S-1583S.
8. Park SK, Jung IC, Lee WK, et al. A combination of green tea extract and l-theanine improves memory and attention in subjects with mild cognitive impairment: a double-blind placebo-controlled study. J Med Food. 2011;14(4):334-343.
9. Feng L, Gwee X, Kua EH, Ng TP. Cognitive function and tea consumption in community dwelling older Chinese in Singapore. J Nutr Health Aging. 2010;14(6):433-438.
10. Amtul Z, Uhrig M, Rozmahel RF, Beyreuther K. Structural insight into the differential effects of omega-3 and omega-6 fatty acids on the production of Aβ peptides and amyloid plaques. J Biol Chem. 2011;286(8):6100-6107.
11. Cole GM, Frautschy SA. DHA may prevent age-related dementia. J Nutr. 2010;140(4):869-874.
12. Jicha GA, Markesberry WR. Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clin Interv Aging. 2010;5:45-61.
13. Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease:
a randomized trial. JAMA. 2010;304(17):1903-1911.
14. Froud KE, Wardhaugh T, Banks D, Saffrey MJ, Stewart MG. Colostrinin alleviates amyloid-β induced toxicity in rat primary hippocampal cultures. J Alzheimers Dis. 2010;20(2):423-426.
15. Janusz M, Zabłocka A. Colostral proline-rich polypeptides: immunoregulatory properties and prospects of therapeutic use in Alzheimer’s disease. Curr Alzheimer Res. 2010;7(4):323-333.
16. Leszek J, Inglot AD, Janusz M, et al. Colostrinin proline-rich polypeptide complex from ovine colostrum: a long-term study of its efficacy in Alzheimer’s disease. Med Sci Monit. 2002;8(10):PI93-P196.
17. Bilikiewicz A, Gaus W. Colostrinin (a naturally occurring, proline-rich, polypeptide mixture) in the treatment of Alzheimer’s disease J Alzheimers Dis. 2004;6(1):17-26.
18. Malouf R, Areosa Sastre A. Vitamin B12 for cognition [update in Cochrane Database Syst Rev. 2009;(1):CD004326]. Cochrane Database Syst Rev. 2003;(3):CD004326.
19. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514.
20. Tseng CN, Gau BS, Lou MF. The effectiveness of exercise on improving cognitive function in older people: a systematic review. J Nurs Res. 2011;19(2):119-131.