Rick Liva, RPh, ND
Why write about quality assurance of natural products? My intent for these quality assurance articles is simple: I will do my best to educate healthcare practitioners about the quality assurance and quality control procedures necessary to make the purest, highest-quality and most effective nutritional supplements and natural medicines. While I realize that “efficacy” is not a direct result of a high-quality product, I can say with certainty that clinical outcome can be influenced by a poor-quality product.
It is essential to draw attention to the many quality assurance holes in the dietary-supplement and natural product supply chain and manufacturing arena. I believe that it is important to have a serious ongoing discussion about significant quality assurance problems with these products that can, and probably do, adversely affect our friends, families and patients. The overall intent of providing this information is to give a detailed presentation of solutions to these quality assurance issues, so that collectively we can increase the integrity of the supply chain.
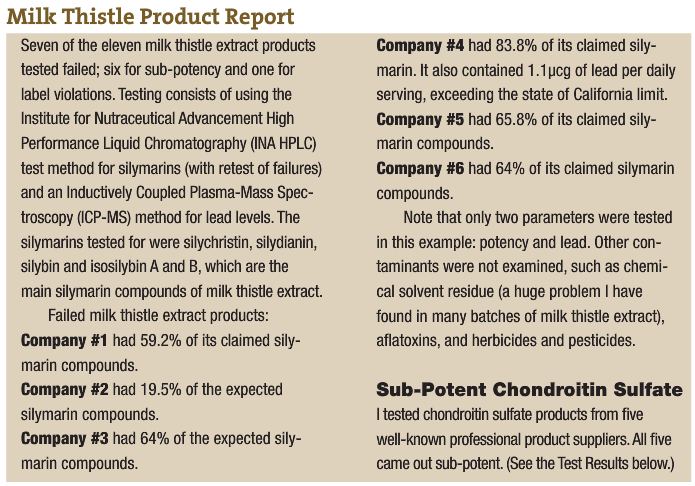
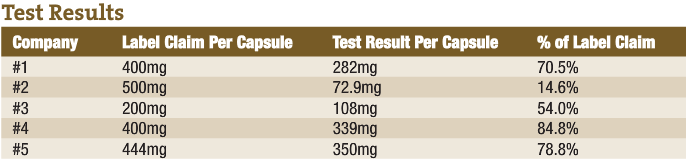
An example of poor product integrity was published recently by a firm that tests products off the shelf. The report (in the accompanying sidebar) shows what they found when they looked at milk thistle extract (one of the products that flunked the test was produced by a professional products company.) The second report is from testing I had performed on chondroitin sulfate. I have seen this kind of product deficiency time and again.
Lab and Lab Method Used
I used one of the largest and most trusted labs in the world currently doing testing on dietary supplements. The lab method used was an official United States Pharmacopeia (USP) method. As far as I know, this method is one of the most accurate and reliable methods for the quantification of chondroitin sulfate. Some manufacturing companies may challenge the use of this lab method, claiming that others may give a higher quantification of chondroitin sulfate. The USP method was used because it is an official method well accepted in the industry.
I believe there is a great deal of poor-quality product in the marketplace. What none of us knows is how many products, the degree of product deficiency and which companies are irresponsible about doing the necessary quality assurance testing needed to provide an authentic, potent and pure product. Why don’t we know? No one is looking on a broad scale. Until now, there was no “specific legal guideline” that the industry had to follow, and no watchdog.
In June, the FDA finalized and put into law manufacturing guidelines for this industry to follow. It is a great step to reduce low-quality product in this industry. However, it also muddies the water. Why? It is very easy for a manufacturer to say it is following all of the rules and producing good-quality product. But, how does a physician know that is the case? In my experience, a large number of cheaters exist in this industry, and they are hard to identify without the tools to do so. I hope to give you some of those tools.
The rules the FDA published are called current Good Manufacturing Practices (cGMPs). The cGMPs are legally binding, and dietary supplement manufacturers must follow them. It is my sincere hope that the FDA enforces them, so everyone is measured by the same yardstick.
Why cGMPs are Needed
cGMP regulations for dietary ingredients and dietary supplements are necessary to promote and protect public health and safety. Unlike other major product areas, there have been no FDA regulations specific to dietary ingredients and dietary supplements that establish a minimum standard of practice for manufacturing, packaging or holding. Now that the FDA has finally published dietary supplement cGMPs, it will help to accomplish the following:
- Establish a level playing field for the industry, which would help prevent irresponsible firms from making and selling adulterated products
- Ensure that every dietary supplement on the market has the safety, identity, purity and strength it purports on the label
- Require that manufacturers have written procedures and documented evidence that their manufacturing process is quality controlled on a consistent basis
- Require manufacturers to test dietary ingredients, particularly botanicals, for identity, heavy metals, pesticides and other contaminants
- Ensure that dietary supplements are produced using a master formula procedure and within a sanitary facility.
After practicing in a cGMP manufacturing environment for the past several years, I am convinced it is critical to ensure the manufacture of high-quality products. Now that adhering to cGMPs is the law, physicians must have some sort of reputable independent audit and certification that proves that companies are in fact adhering to the cGMP guidelines. The FDA is spread thin and will have very limited means to enforce these rules. Therefore, an independent audit and asking for proof of quality assurance verification becomes our best evidence of ensuring compliance with cGMPs. In a future column, I’ll provide questions a clinician can ask a company to determine its quality practices, a sort of mini-audit designed to prove quality assurance practices.
 Please remember: The key and most important factor to producing a natural product that is authentic, meets potency claims and has maximum freedom from contamination is verification testing. If a company is not comprehensively testing its raw materials and finished products, there is no possible way to consistently achieve authenticity, potency claims and maximum freedom from contamination. Are you buying from a professional products company that does no testing? I know of several and I am sure there are more. How did I find out? I asked. When physicians don’t ask, suppliers get away with poor quality, they are not held accountable and the beat goes on. Remember this the next time you prescribe and sell products from your office, and ask yourself if you have done the due diligence regarding the level of quality assurance your vendors provide.
Please remember: The key and most important factor to producing a natural product that is authentic, meets potency claims and has maximum freedom from contamination is verification testing. If a company is not comprehensively testing its raw materials and finished products, there is no possible way to consistently achieve authenticity, potency claims and maximum freedom from contamination. Are you buying from a professional products company that does no testing? I know of several and I am sure there are more. How did I find out? I asked. When physicians don’t ask, suppliers get away with poor quality, they are not held accountable and the beat goes on. Remember this the next time you prescribe and sell products from your office, and ask yourself if you have done the due diligence regarding the level of quality assurance your vendors provide.
In the next column, I will highlight and summarize exactly what manufacturing companies must do to comply with the new FDA cGMPs. Armed with that information, you can become a force to drive companies to produce quality products. Start asking and evaluating the data they provide. Vote with your dollars, which is the force you have to stimulate change.
 Rick Liva, RPh, ND is the managing physician at the Connecticut Center for Health in Middletown, Conn. He is a founding member of the American Association of Naturopathic Physicians (AANP) and past president of the Connecticut Society of Naturopathic Physicians. He has been involved in dietary supplements manufacturing since 1985 and is the president, CEO and director of quality control and quality assurance at Vital Nutrients, certified by NSF International and the Natural Products Association (NPA) in cGMPs for dietary supplements.
Rick Liva, RPh, ND is the managing physician at the Connecticut Center for Health in Middletown, Conn. He is a founding member of the American Association of Naturopathic Physicians (AANP) and past president of the Connecticut Society of Naturopathic Physicians. He has been involved in dietary supplements manufacturing since 1985 and is the president, CEO and director of quality control and quality assurance at Vital Nutrients, certified by NSF International and the Natural Products Association (NPA) in cGMPs for dietary supplements.
Reference
USP: Chondroitin Sulfate by CPC (29th revision). US Pharmacopeia Convention Inc., 2006, Rockville, p. 2306.





