Heather Paulson, ND, FABNO
The number of adults who have survived childhood cancer is rapidly increasing. The likelihood that NDs will be responsible for monitoring the late effects of cancer treatment is also increasing. This article reviews the common complications, symptoms, and monitoring tools for this unique patient population.
Every year, approximately 20,000 children in the United States are diagnosed with cancer. With a cure rate approaching 80%, the number of childhood cancer survivors grows by more than 15,000 each year.1,2 Currently, 1 in 450 individuals is a childhood cancer survivor.3 A 2003 study by Oeffinger revealed that 1 in 3 survivors remains free of late effects from cancer treatment, implying that the other two-thirds will suffer from chronic and potentially life-threatening complications.4 Late effects are defined as the long-term side effects from radiation, surgery, or chemotherapy with an onset following the completion of treatment. Some late effects can take up to 30 years to manifest.
The late effects of cancer treatment can affect a survivor’s physical, mental, and psychosocial health. Survivors at the highest risk for late effects are those who as children were treated for bone tumors, central nervous system tumors, and Hodgkin’s lymphoma.4 The risks of late effects do not plateau or decline over time and survivors remain at risk their entire life.4 Providing appropriate healthcare and screenings for survivors of childhood cancers can be challenging, but a proactive approach reduces the associated risks and life-threatening complications.5
Late Effects
Skeletal
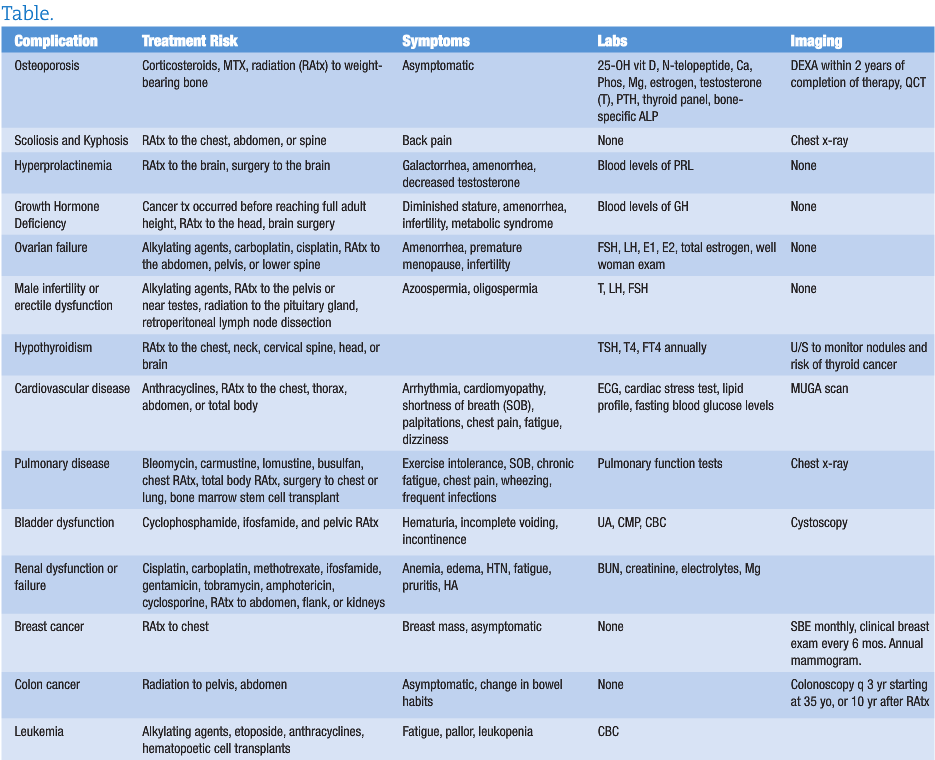
Due to the effects of corticosteroids, methotrexate, and radiation therapy on weight-bearing bones, childhood cancer survivors are at an increased risk for osteoporosis. Secondary causes of decreased bone mineral density in childhood cancer survivors include low levels of estrogen or testosterone, growth hormone deficiency, hyperthyroidism, and prolonged periods of bed rest. It is important to educate childhood cancer survivors on the importance of weight-bearing exercise, appropriate levels of vitamin D, and daily intake of calcium-rich foods and other bone-building nutrients. A baseline DEXA scan is recommended 2 or more years after completion of therapy.6 Other monitoring options include quantitative computed tomography (QCT), N-telopeptide, calcium, phosphorus, magnesium, estrogen, testosterone, parathyroid hormone, FT4, T4, TSH, and bone-specific alkaline phosphatase.7
Survivors of childhood cancers are at increased risk of developing scoliosis and kyphosis, particularly if they had radiation to the trunk; combined surgery and radiation to the chest, abdomen or spine; or the tumor was near the spine.8 Diagnosis of scoliosis or kyphosis may be detected on physical exam or x-ray. Treatment can include bracing or surgery.
An additional late skeletal effect is osteonecrosis. Symptoms of unresolved joint pain that is aggravated by motion and rest should be investigated with an x-ray, CT, MRI, or bone scan.9
Endocrine
Radiation to the pituitary gland, brain surgery, secondary cancers, and multiple medications can cause long-term adverse effects in the hypothalamic-pituitary-adrenal (HPA) axis. Common late effects include hyperprolactinemia, hypopituitarism, and central adrenal insufficiency.10 Hyperprolactinemia can affect both men and women, with symptoms of galactorrhea, irregular or absent menstrual periods in women, and decreased testosterone in men. Screening blood levels of prolactin can dictate further treatment if needed.
The risk of growth hormone deficiency is increased if the cancer treatment occurred before reaching adult height, if the treatment included radiation to any area of the head, total body irradiation, or after cranial surgery.11 Growth screening should occur once every 6 months until the child reaches puberty. If any concerns are raised during physical exam, evaluation for growth hormone insufficiency is recommended. Treatment options include synthetic growth hormone and may be required well into adulthood.
Central adrenal insufficiency risk increases if they were treated with brain surgery or brain radiation. Symptoms include fatigue, weakness, poor appetite, and dizziness. Screening recommendations include annual cortisol screening for 15 years after radiation therapy.12
Reproductive Complications
Certain treatments affect the ovaries and the reserve supply of eggs. If damage occurs, early ovarian failure and early menopause ensue. Common causes of ovarian failure include treatment with alkylating agents such as carboplatin or cisplatin, oophorectomy, and radiation to the abdomen, pelvis, lower spine or region of the pituitary gland. Monitoring can include FSH, LH, estradiol, estrone, total estrogen, and regular gynecologic examinations.13,14
For men, cancer treatment can cause deficiency of testosterone, LH, and FSH . Treatments that increase risk of hormonal or functional infertility include alkylating agents, radiation to the pelvis or near the testicles, radiation to or near the pituitary gland, castration, cystectomy, and retroperitoneal lymph node dissection. Monitoring can include blood levels of FSH, LH, and testosterone. If testosterone levels are low, replacement with exogenous testosterone may be warranted. It can take up to 10 years post cancer treatment for azoospermia or oligospermia to resolve.15
Thyroid
Adult childhood cancer survivors who received radiation to the chest, neck, cervical spine, head or brain are at risk for hypothyroidism. Thyroid problems may not occur until adulthood. Monitoring levels of TSH and T4 annually is recommended, particularly for female survivors who are planning to become pregnant. Untreated hypothyroidism can cause birth defects. Childhood cancer survivors are also at an increased risk of thyroid cancer, thyroid nodules, and hyperthyroidism.16
Cardiovascular
While most adult survivors of childhood cancers will not have cardiovascular complications, certain treatments do increase future risk. These treatments include anthracyclines; radiation therapy to the chest, thorax, thoracic spine, or abdomen; and total body irradiation. Anthracylines can cause left ventricular dysfunction, cardiomyopathy, and arrhythmias. Radiation therapy is more likely to cause scarring and stiffening of the heart leading to arrhythmias, cardiomyopathy, coronary artery disease, valvular stenosis or insufficiency, and pericarditis or pericardial fibrosis. Symptoms and complications from late cardiovascular effects can occur up to 30 years after initial cancer treatment.17
Symptoms of cardiovascular disease include shortness of breath, dizziness, severe fatigue, chest pain, perspiration, nausea, vomiting, edema, unresolved cough or wheezing, arrhythmia, and palpitations. Ongoing monitoring may be recommended, especially if as young adults these survivors choose to play high school sports. Performing activities that require heavy lifting or sustained isometric activity (such as wrestling) may increase risk of heart failure. Special caution also needs to be in place during pregnancy and illnesses with high fever.
Monitoring for cardiac complications can be done with electrocardiogram, MUGA scan (multiple-gated acquisition scan), or cardiac stress test. It is recommended to do the baseline test 2 years after completion of treatment and followed up every year to every 5 years depending on age of initial treatment and dose of chest radiation or total anthracycline dose. Specific screening guidelines are provided by the Children’s Oncology Group.18 Lipid profiles and fasting glucose are recommended as a screening tool once every 2 years to check for and modify other cardiac risk factors.
Pulmonary
Several treatments for childhood cancers can cause permanent lung damage. These treatments include bleomycin, carmustine, lomustine, busulfan, chest or total radiation, surgery to the chest or lung, bone marrow or stem cell transplant, and anthracyline chemotherapy. A prior medical history of asthma, lung infection, or exposure to secondhand smoke also increases the risk of late pulmonary effects. Common long-term health problems that develop are pulmonary fibrosis, frequent lung infections, restrictive airway disease, obstructive airway disease, and bronchiolitis obliterans. Symptoms of reduced pulmonary function include exercise intolerance, shortness of breath, chronic fatigue, chest pain, wheezing, and frequent infections. Monitoring includes a chest x-ray and pulmonary function tests performed 2 years after the completion of treatment, with follow-up pulmonary function tests as needed.19
Gastrointestinal
Adult survivors of childhood cancers can present with malabsorption, esophageal strictures, cholelithiasis, hepatic fibrosis, chronic enterocolitis, bowel obstruction, bowel adhesion, and colorectal cancer. These late effects are associated with abdominal and pelvic surgery and radiation to the neck, chest, abdomen or pelvis. Symptoms of late gastrointestinal effects include GERD (gastroesophageal reflux disease), chronic nausea or vomiting, abdominal pain, chronic diarrhea or constipation, changes in weight, abdominal distention, and jaundice. Monitoring should include a comprehensive metabolic panel, stool guaiac test, ultrasound of the gallbladder, and colonoscopy and endoscopy as indicated by clinical symptomatology.20 A comprehensive metabolic panel should be performed annually.
Hepatic
Damage to the liver can be caused by abdominal radiation, liver radiation, methotrexate, mercaptopurine, and thioguanine. People who received blood products prior to 1971 are at an increased risk of hepatitis B, and prior to 1992 have an increased risk of hepatitis C.21 Obtaining viral titers may be recommended if symptoms of jaundice, right upper quadrant pain, or elevated liver function tests are noted.
Urologic
Survivors that were treated with cisplatin, carboplatin, methotrexate, ifosfamide, gentamicin, tobramycin, amphotericin, and cyclosporine are at an increased risk of late kidney damage. Radiation to the abdomen, flank, or total body also increases the risk of renal damage. Signs of chronic renal insufficiency or failure include edema, anemia, hypertension, fatigue, nausea, vomiting, pruritis, and headaches. Monitoring of renal function can include BUN, creatinine, electrolytes, and magnesium.
Bladder function and secondary bladder cancers are increased in people treated with cyclophosphamide, ifosfamide, and pelvic radiation. The most common late effect is hemorrhagic cystitis. Monitoring includes urinary analysis when indicated. Incidence of hemorrhagic cystitis can be reduced by avoiding caffeinated beverages and drinking plenty of fluids. Another late effect can be bladder fibrosis that often presents as urinary incontinence or incomplete bladder emptying. Bladder fibrosis can be diagnosed by cystoscopy.2 Bladder cancer as a late effect most commonly presents as hematuria and suprapubic pain. A thorough urological work up including cystoscopy is recommended to rule out late effects of chemotherapy or pelvic radiation.23
Secondary Cancers
Women treated with radiation to the chest during childhood, adolescence, or young adulthood are at increased risk of developing breast cancer. They tend to develop breast cancer at a younger age and risk increases throughout their lifetime.24 Monitoring should include monthly self-breast exams and annual clinical breast exam until age 25. After reaching 25 years of age, clinical breast exam should occur every 6 months. Yearly mammograms and breast MRI should start at age 25, or 8 years after radiation (whichever occurs last).
Colon cancer risk is increased if radiation to the abdomen, pelvis, or spine was performed.25 If children received radiation doses of 30 Gy or higher, a colonoscopy should be performed every 3 years starting at age 35, or 10 years after radiation therapy (whichever occurs last).
Secondary leukemia usually occurs within 10 years after treatment for the original cancer. An increased risk of secondary leukemia is seen in people who were treated with high doses of alkylating agents, epipodophyllotoxins (eg, etoposide or teniposide), anthracylines, and hematopoietic cell transplants.25
A secondary solid tumor is most likely to occur in someone that received radiation therapy, particularly if radiation was at a young age and a large field was used. The most common sites for secondary solid tumors include the skin, breast, spine, brain, thyroid, and bones. Secondary solid tumors tend to occur 10 or more years after initial cancer treatment.25
As childhood cancer survival rates continue to improve, NDs will become an important source for continued monitoring of late effects. The symptoms of late effects can vary between gastrointestinal upset, to chest pain, to endocrine disruption. By actively monitoring adult survivors of childhood cancers, potentially life-threatening late effects can be caught and avoided. NDs can also educate survivors on risk-reducing behaviors such as eating a vegetable and whole grain-based diet, exercising, and promoting necessary supplementation when indicated.
The Oncology Association of Naturopathic Physicians (OncANP) was founded in 2004 to enhance the quality of life of people living with cancer through both increasing the collaboration between NDs working with patients with cancer and integrating naturopathic practice into medical oncology care. With its coorganization, the American Board of Naturopathic Oncology (ABNO), the OncANP has set standards, instituted testing and now credentials Fellows in Naturopathic Oncology.
The OncANP welcomes all NDs interested in improving their knowledge and ability to work with oncology patients. For more information see www.OncANP.org.
 Heather Paulson, ND graduated from the University of California – Santa Barbara with a BS in aquatic biology. Dr. Paulson pursued her doctorate in naturopathic medicine at SCNM. Currently, Dr. Paulson is completing a two-year residency focusing on naturopathic oncology at the Center for Cancer Care in Goshen, Ind. This integrative medical facility is part of Goshen General Hospital and includes a team of integrative practitioners including radiation, medical and surgical oncologists; naturopathic physicians; dietitians; and psychoneuroimmunologists. Goshen Center for Cancer Care was recently recognized with the national HOPE Award for integration of care by Hematology & Oncology News & Issues magazine.
Heather Paulson, ND graduated from the University of California – Santa Barbara with a BS in aquatic biology. Dr. Paulson pursued her doctorate in naturopathic medicine at SCNM. Currently, Dr. Paulson is completing a two-year residency focusing on naturopathic oncology at the Center for Cancer Care in Goshen, Ind. This integrative medical facility is part of Goshen General Hospital and includes a team of integrative practitioners including radiation, medical and surgical oncologists; naturopathic physicians; dietitians; and psychoneuroimmunologists. Goshen Center for Cancer Care was recently recognized with the national HOPE Award for integration of care by Hematology & Oncology News & Issues magazine.
References
- Hewitt ME, Weiner SL, Simone JV, eds. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: The National Academies Press; 2003.
- Rosoff PM. The two-edged sword of curing childhood cancer. N Engl J Med. 2006;355(15):1522-1523.
- Meadows AT. Pediatric Cancer Survivors: Past History and Future Challenges. December 2001. National Cancer Policy Board, Institute of medicine.
- Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27(3):143-167.
- Oeffinger KC, Nathan PC, Kremer LC. Challenges after curative treatment of childhood cancer and long-term follow up of survivors. Hematol Oncol Clin North Am. 2010;24(1):129-149.
- Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):705E-713E.
- Rai SN, Hudson MM, McCammon E, et al. Implementing an intervention to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia: BONEII, a prospective placebo-controlled double-blind randomized interventional longitudinal study design. Contemp Clin Trials. 2008;29(5):711-719.
- Parisi MT, Fahmy JL, Kaminsky CK, Malogolowkin MH. Complications of cancer therapy in children: a radiologist’s guide. Radiographics. 1999;19(2):283-297.
- Kaste SC. Skeletal toxicities of treatment in children with cancer. Pediatr Blood Cancer. 2008;50(suppl 2):460-473.
- Rutter MM, Rose SR. Long-term endocrine sequelae of childhood cancer. Curr Opin Pediatr. 2007;19(4):480-487.
- Gleeson HK, Shalet SM. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr Relat Cancer. 2004;11(4):589-602.
- Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, Lange M, Poulsen HS, Müller J. Assessment of the hypothalamo-pituitary-adrenal axis in patients treated with radiotherapy and chemotherapy for childhood brain cancer. J Clin Endocrinol Metab. 2003;88(7):3149-3154.
- Green DM, Sklar CA, Boice JD Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2374-2381.
- Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98(13):890-896.
- Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(2):332-339.
- Mandanat LM, Lähteenmäki PM, Hurme S, Dvba T, Salmi TT, Sankila R. Hypothyroidism among pediatric cancer patients: a nationwide, registry-based study. Int J Cancer. 2008;122(8):1868-1872.
- Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28(8):1308-1315.
- Shankar SM, Marina N, Hudson MM, et al; Cardiovascular Disease Task Force of the Children’s Oncology Group. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121(2):387E-396E.
- Dickerman JD. The late effects of childhood cancer therapy. Pediatrics. 2007;119(3):554-568.
- Landier W, Bhatia S. Cancer survivorship: a pediatric perspective. Oncologist. 2008;13(11):1181-1192.
- Castellino S, Muir A, Shah A, et al. Hepato-biliary late effects in survivors of childhood and adolescent cancer: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2010;54(5):663-669.
- Shnorhavorian M, Friedman DL, Koyle MA. Genitourinary long-term outcomes for childhood cancer survivors. Curr Urol Rep. 2009;10(2):134-137.
- Frobisher C, Gurung PM, Leiper A, et al. Risk of bladder tumours after childhood cancer: The British Childhood Cancer Survivor Study [published online ahead of print February 22, 2010]. BJU Int. doi: 10.1111/j.1464-410X.2010.09224.x.
- Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152(7):444-455.
- Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2356-2362.