Vis Medicatrix Naturae
Carrie Decker, ND
As medical research continues to grow, so too does our understanding of the extracellular matrix (ECM) and its dynamic and systemic effects. Once thought to be only a backdrop to the activities of the cells found within it, we now know the ECM has complex means of interacting with the rest of the body, much like adipose tissue.1 Molecules within the ECM, such as collagen, elastin, and proteoglycans, interact with the cells, influencing cellular signaling,2,3 cellular differentiation,4 and even immune system function.5
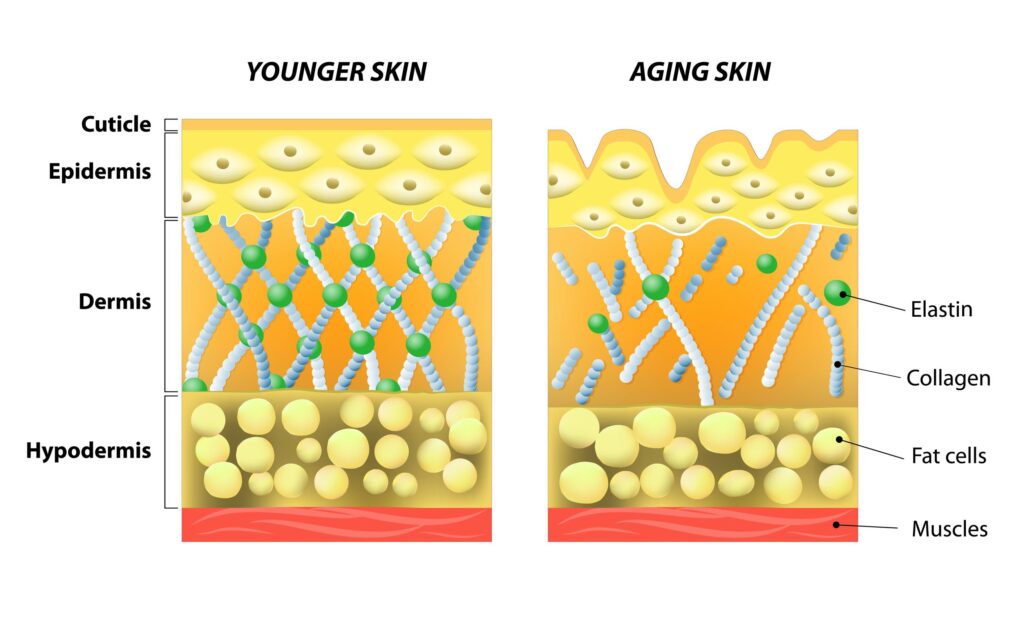
Two families of molecules critical to the structure and function of the ECM are collagen and proteoglycans.6,7 Collagen provides a structural backbone to the ECM,8 while proteoglycans interact with collagen and, as highly hydrophilic molecules, retain water within the ECM. In cartilage, type II collagen is the primary structural backbone of the ECM, with many large proteoglycans – known as aggrecans – interacting with the collagen fibril network and hyaluronic acid.9,10 These aggrecans contain covalently-bound chondroitin sulfate and keratan sulfate chains, collectively known as glycosaminoglycans. In the skin, type I collagen prevails, with type III collagen also being a significant fraction.11 As with cartilage, the proteoglycans found in the skin structurally interact with collagen and affect the skin’s functional properties and structural integrity, in part by retaining moisture within the tissue.12
With aging, functional changes in the ECM occur in both cartilage and skin. Chondrocytes have minimal ability to replicate, and their ability to synthesize collagen and proteoglycans declines with age.13 Similarly, in the skin the number of fibroblasts, as well as their ability to produce these molecules, declines dramatically with age: by age 80, their capacity is only 10-25% of that of fibroblasts sourced from fetal human skin, while the quantity of fibroblasts decreases significantly as well.14,15
Proteoglycans: Bone Broth of the Future
Collagen has long been used as an oral supplement for its joint- and skin-supportive properties. Numerous scientific studies exist, with collective findings suggesting that oral collagen supplementation supports tissue regeneration and reduces joint pain, bone density loss, and skin aging.16 Although collagen as a supplement has considerable evidence for its use, proteoglycans are fast receiving interest, given the array of clinical studies showing their benefits. Until recently, techniques did not exist for large-scale extraction of proteoglycans in their natural, unaltered state from animal products. However, recent technological developments enable the safe extraction of proteoglycans from otherwise-wasted byproducts of the food chain, such as animal cartilage.17
Salmon nasal cartilage is one source of proteoglycans that has been highly researched with positive findings in a myriad of settings. Salmon nasal cartilage proteoglycans (SNCPs) have functional domains and an amino acid composition very similar to the primary proteoglycan, aggrecan, that is found in the articular cartilage of mammals.18
Effects on Immunity, Inflammation & Arthritis
Similar to the immunomodulatory and oral tolerance effects seen with low doses of type II collagen,19 oral proteoglycan supplementation has been suggested to have anti-inflammatory effects via mechanisms related to immune tolerance.20 In several animal models of autoimmune disease, supplementation with proteoglycans has been shown to induce a regulatory T-cell response and/or to reduce disease progression.
For example, in a mouse model of ulcerative colitis (UC), mice that received water containing SNCPs had a significantly increased survival rate compared to controls.21 A subsequent animal UC model investigated the mechanisms via which SNCPs may have this effect, finding that daily oral administration of SNCPs attenuated progression of colitis and suppressed disease-related inflammatory cytokine levels.22 This was shown to be through the enhanced induction of regulatory T-cells via the Foxp3 gene, a master controller of the regulatory T-cell response.23 A similar response was seen in experimental autoimmune encephalomyelitis, a common mouse model of multiple sclerosis, including reduced symptom severity and enhanced Foxp3 expression in mice treated once daily with oral SNCPs.24 Finally, in a mouse model of rheumatoid arthritis (RA) (collagen-induced RA), daily oral administration of SNCPs significantly attenuated clinical severity and histological changes via reductions in macrophage and neutrophil invasion as well as levels of inflammatory cytokines and chemokines.25
Anti-inflammatory effects with oral proteoglycan therapies have also been seen in other settings. The inflammatory state associated with type 2 diabetes and obesity, induced by high-fat feeding, has been shown to decrease in mice with oral supplementation of SNCPs.26 Supplementation with SNCPs reduced the expression of tumor necrosis factor-alpha (TNFα), interleukin (IL)-6, and the proportion of M1 macrophages that contribute to proinflammatory response and the development of insulin resistance.27 Along with these changes, reductions in both fasting insulin levels and glucose-stimulated hyperglycemia were observed in the mice receiving SNCPs.
Mechanistically, some of the benefits of proteoglycans may also be mediated via their effects on the gut flora. Oral supplementation with proteoglycans has been shown to modulate the balance of bacteria in the gut, increasing lactobacilli and other beneficial, immunomodulatory bacteria and decreasing levels of several bacteria associated with disease.28
Although oral proteoglycan therapies have not yet been studied in human conditions of autoimmunity, they have been shown to have anti-inflammatory effects in clinical studies of osteoarthritis, another condition with inflammation as an underlying factor.29–31 Multiple randomized, placebo-controlled studies have investigated the impact of proteoglycans in individuals with discomfort of the knee, finding that a very low dose of 5-10 mg significantly improves scores related to joint mobility and pain.20,32 At a dosage of 5 mg daily, a trend of reduction in high-sensitivity C-reactive protein (hs-CRP) levels was also seen, paralleling the improvements in Visual Analogue Scale (VAS) comprehensive scores. Significantly reduced collagen breakdown was also seen clinically at a dosage of 10 mg daily.33
Proteoglycans & Skin Aging
The impact of SNCPs on skin health has been assessed in cellular, animal, and human studies. With aging, the composition of proteoglycans in the skin dramatically shifts, leading to diminished hydration, a loss of skin viscoelasticity, and altered wound healing.34-36 Although important to many of us cosmetically, these changes also contribute to non-healing pressure sores (also known as bed sores or pressure ulcers),37 infections,38 and related complications in the aging population.
In fibroblast monolayer studies, the introduction of a solution containing SNCPs was shown to stimulate cell migration and proliferation.39 In a subsequent animal model, compared to controls, topical application of SNCPs was shown to accelerate the closure of 2 types of wounds, one being infected and the other not, with an increase in transforming growth factor-beta (TGFβ) (which promotes re-epithelialization).40 Additionally, application of SNCPs modulated the immune and inflammatory responses and decreased bacterial counts in the infected wounds. Animal studies suggest SNCPs also may protect the skin from the damage caused by the ultraviolet (UV) rays of the sun. In animals exposed to UV-B light, those treated with SNCPs experienced a reduction in erythema, transepidermal water loss, inflammatory cytokine levels, and improved skin hydration compared to controls after 4 and 7 weeks of repeated UV-B exposure.41
In human clinical studies, improvements in skin appearance and tissue quality have also been seen in subjects taking low does of oral proteoglycans. In a randomized, double-blind, placebo-controlled study of healthy individuals with a mean age of 39 to 40 years old, 5 mg of proteoglycans taken daily for a period of 2 weeks significantly increased skin viscoelasticity, recovery after deformation, and skin hydration (as evaluated by skin conductance), and decreased skin looseness and roughness (as evaluated by skin micrographs).42 Improvements were also evident visually, with a significant decrease in wrinkles, conspicuous facial pores, and blotches in the individuals taking proteoglycans. Given that cellular turnover in the skin takes approximately 28 days, one would expect benefits to be even more dramatic after a longer period of use.
Conclusion
Although the changes with aging will never cease to challenge our health in numerous ways, the positive findings from using minimally invasive interventions such as proteoglycans offer hope to those struggling with age-related joint and skin changes. As research surrounding this newly available therapy advances, there likely will be additional studies in settings of autoimmune disease that also may pique further interest. Additionally, as we further understand the role the extracellular matrix plays in other facets of health and disease,43 research investigating the impact of proteoglycans on other aspects of health may soon follow.
References:
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209(2):139-151.
- Ramirez F, Rifkin DB. Cell signaling events: a view from the matrix. Matrix Biol. 2003;22(2):101-107.
- Duca L, Floquet N, Alix AJ, et al. Elastin as a matrikine. Crit Rev Oncol Hematol. 2004;49(3):235-244.
- Hoshiba T, Chen G, Endo C, et al. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016;2016:6397820.
- Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67(2):149-159.
- Bornstein P, Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957-1003.
- Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11-55.
- Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531-1546.
- Poole AR, Kobayashi M, Yasuda T, et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61 Suppl 2:ii78-81.
- Kiani C, Chen L, Wu YJ, et al. Structure and function of aggrecan. Cell Res. 2002;12(1):19-32.
- Weber L, Kirsch E, Müller P, et al. Collagen type distribution and macromolecular organization of connective tissue in different layers of human skin. J Invest Dermatol. 1984;82(2):156-160.
- Smith MM, Melrose J. Proteoglycans in normal and healing skin. Adv Wound Care (New Rochelle). 2015;4(3):152-173.
- Hou A, Chen P, Tang H, et al. Cellular senescence in osteoarthritis and anti-aging strategies. Mech Ageing Dev. 2018 Aug 11. pii: S0047-6374(18)30062-9.
- Takeda K, Gosiewska A, Peterkofsky B. Similar, but not identical, modulation of expression of extracellular matrix components during in vitro and in vivo aging of human skin fibroblasts. J Cell Physiol. 1992;153(3):450-459.
- Tigges J, Krutmann J, Fritsche E, et al. The hallmarks of fibroblast ageing. Mech Ageing Dev. 2014;138:26-44.
- Figueres Juher T, Basés Pérez E. An overview of the beneficial effects of hydrolyzed collagen intake on joint and bone health and on skin aging. Nutr Hosp. 2015;32 Suppl 1:62-6. [Article in Spanish]
- Takahashi T, Matsubara M, Fujita J, et al. Safety evaluation of highly purified proteoglycan industrially extracted from salmon nasal cartilage. Pharmacometrics. 2015;89(1/2):15-22.
- Kakizaki I, Tatara Y, Majima M, et al. Identification of proteoglycan from salmon nasal cartilage. Arch Biochem Biophys. 2011;506(1):58-65.
- Barnett ML, Kremer JM, St Clair EW, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41(2):290-297.
- Kuriyama Y, Yoshida Y. Efficacy of dietary supplement contained proteoglycan extracted from salmon nasal cartilage on knee uncomfortableness in healthy volunteers. Jpn Pharmacol Ther. 2017;45(11):1795-1808.
- Majima M, Takagaki K, Sudo SI, et al. Effect of proteoglycan on experimental colitis. International Congress Series. 2001;1223:221-224.
- Mitsui T, Sashinami H, Sato F, et al. Salmon cartilage proteoglycan suppresses mouse experimental colitis through induction of Foxp3+ regulatory T cells. Biochem Biophys Res Commun. 2010;402(2):209-215.
- Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16(11):1643-1656.
- Sashinami H, Asano K, Yoshimura S, et al. Salmon proteoglycan suppresses progression of mouse experimental autoimmune encephalomyelitis via regulation of Th17 and Foxp3(+) regulatory T cells. Life Sci. 2012;91(25-26):1263-1269.
- Yoshimura S, Asano K, Nakane A. Attenuation of collagen-induced arthritis in mice by salmon proteoglycan. Biomed Res Int. 2014;2014:406453.
- Hirose S, Asano K, Nakane A. Attenuation of obesity-induced inflammation in mice orally administered with salmon cartilage proteoglycan, a prophylactic agent. Biochem Biophys Res Commun. 2017;484(3):480-485.
- Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14(12):1225-1230.
- Asano K, Yoshimura S, Nakane A. Alteration of intestinal microbiota in mice orally administered with salmon cartilage proteoglycan, a prophylactic agent. PLoS One. 2013;8(9):e75008.
- Spector TD, Hart DJ, Nandra D. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40(4):723-727.
- Sharif M, Shepstone L, Elson CJ, et al. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59(1):71-74.
- Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471478.
- Najima M, Munekata M, Soeda Y. Usefulness of the supplement containing proteoglycan for Japanese healthy people feeling knee’s discomfort. Shinryo to Shinyaku (Med Cons New-Remed). 2016;53(3):228-236.
- Tomonaga A, Takahashi T, Tanaka YT, et al. Evaluation of the effect of salmon nasal proteoglycan on biomarkers for cartilage metabolism in individuals with knee joint discomfort: A randomized double-blind placebo-controlled clinical study. Exp Ther Med. 2017;14(1):115-126.
- Carrino DA, Sorrell JM, Caplan AI. Age-related changes in the proteoglycans of human skin. Arch Biochem Biophys. 2000;373(1):91-101.
- Röck K, Fischer JW. Role of the extracellular matrix in extrinsic skin aging. Hautarzt. 2011;62(8):591-597. [Article in German]
- Maquart FX. Extracellular matrix: a major partner of wound healing. Bull Acad Natl Med. 2015;199(7):1199-1209.
- Bouten CV, Oomens CW, Baaijens FP, Bader DL. The etiology of pressure ulcers: skin deep or muscle bound? Arch Phys Med Rehabil. 2003;84(4):616-619.
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219-229.
- Ito G, Kobayashi T, Takeda Y, Sokabe M. Proteoglycan from salmon nasal cartridge [corrected] promotes in vitro wound healing of fibroblast monolayers via the CD44 receptor. Biochem Biophys Res Commun. 2015;456(3):792-798.
- Hirose S, Narita K, Asano K, Nakane A. Salmon cartilage proteoglycan promotes the healing process of Staphylococcus aureus-infected wound. Heliyon. 2018;4(3):e00587.
- Goto M, Yamazaki S, Kato Y, et al. Anti-aging effects of high molecular weight proteoglycan from salmon nasal cartilage in hairless mice. Int J Mol Med. 2012;29(5):761-768.
- Takahashi T, Matsubara J, Wakamatsu K, et al. Ingestion of salmon nasal cartilage-derived proteoglycan improves skin condition: A randomized, double-blind, controlled study. Immun Endoc Metab Agents Med Chem. 2015;15(2):160-167.
- Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786-801.
 Carrie Decker, ND, graduated with honors from the National College of Natural Medicine (now the National University of Natural Medicine) in Portland, OR. Prior to becoming a naturopathic physician, Dr Decker was an engineer and obtained graduate degrees in biomedical and mechanical engineering from the University of Wisconsin-Madison and University of Illinois at Urbana-Champaign, respectively. She continues to enjoy academic research and writing and uses these skills to support integrative medicine education as a writer and contributor to various resources. Dr Decker supports Allergy Research Group as a member of their education and product development team.
Carrie Decker, ND, graduated with honors from the National College of Natural Medicine (now the National University of Natural Medicine) in Portland, OR. Prior to becoming a naturopathic physician, Dr Decker was an engineer and obtained graduate degrees in biomedical and mechanical engineering from the University of Wisconsin-Madison and University of Illinois at Urbana-Champaign, respectively. She continues to enjoy academic research and writing and uses these skills to support integrative medicine education as a writer and contributor to various resources. Dr Decker supports Allergy Research Group as a member of their education and product development team.