Anna Garber, MSc
Monique Aucoin, ND
Sukriti Bhardwaj, BHSc
Tolle Causam
The field of nutritional psychiatry is relatively recent but is gaining momentum in terms of its evidence base and public interest.1 Several studies suggest a relationship between improved nutrition and better mental health outcomes. Epidemiological studies, including a recent meta-analysis, have shown that a diet including fruits, vegetables, fish, olive oil, nuts, and legumes may reduce the chance of developing depression,2 and a recent double-blind RCT demonstrated a significant improvement in depression severity with an adjunctive dietary intervention.3 Many mechanisms have been studied with respect to the role of diet in mental health and illness, including micronutrients such as vitamins and minerals,4 the type of dietary fat,5 the impact of food on the microbiome,6 and the influence of dietary sugar.7, 8 Sugar in the diet is thought to impact mental health through several pathways including indirect ones, such as an influence on the gut microbiome.9 In this paper, we will be reporting the evidence for the direct impact of dietary sugar on the brain via its manipulation of blood glucose levels, as well as presenting information on possible mechanisms, presenting a clinical case, and sharing our clinical recommendations.
What is Glycemic Load?
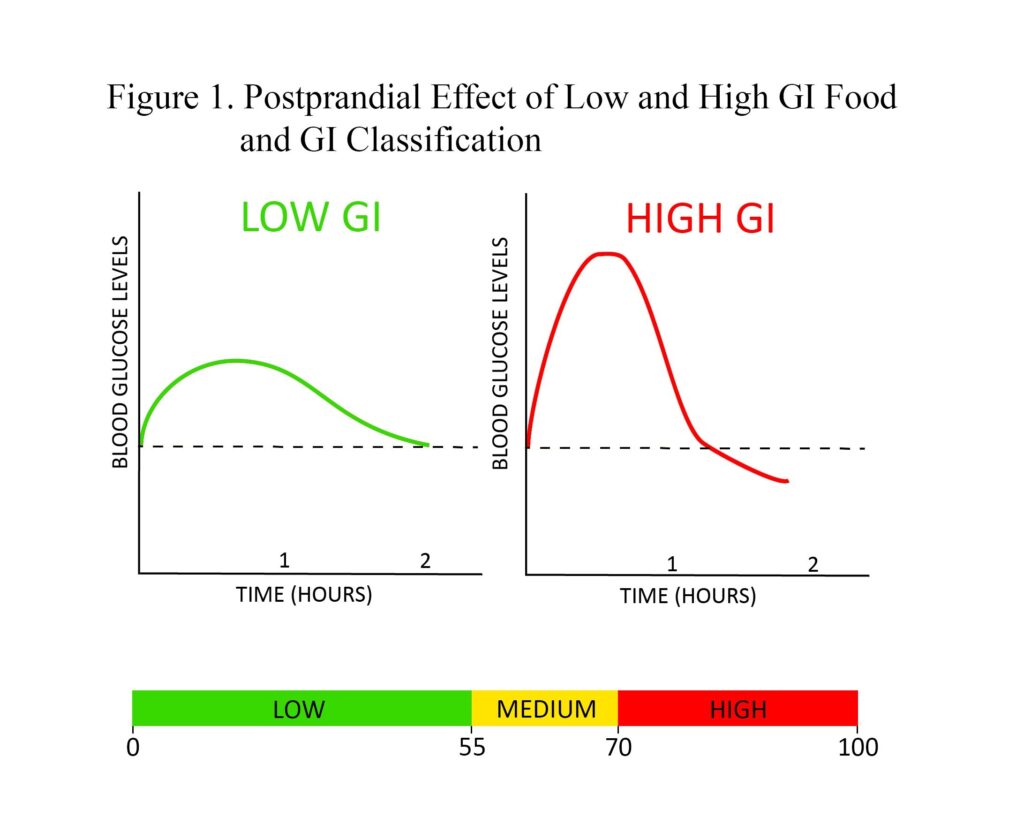
Before defining glycemic load, one must understand the concept of glycemic index (GI). GI is a measure of the postprandial effect of a carbohydrate-containing food on blood glucose levels. Low-GI foods produce a gradual rise and longer-sustained levels of blood glucose after consumption compared to high-GI foods, which generate a spike followed by a fast reduction that can dip below baseline blood glucose levels (Figure 1). GI can be used to assess dietary carbohydrate quality; however, it is limited by the fact that GI values are based on consuming a serving of food that contains a specific amount of available carbohydrates (usually 50 grams). Thus, assessing diet with GI tends to limit its value, as portion sizes vary drastically between individuals and would therefore elicit different glycemic responses. This is where glycemic load becomes useful.
Glycemic load (GL) is a weighted measure of GI that is calculated by multiplying a food’s GI by the number of grams of available carbohydrate in the food’s serving, then dividing by 100. GL is a more useful characterization of carbohydrate consumption because it accounts for both the quality (GI) and quantity (grams) of consumed carbohydrates. GL should be assessed using total daily dietary intake. As carbohydrate consumption can vary among diets differing in calories, GL/1000 cal has been established to be the best predictor of the glycemic response of foods.10 It is important to note that both GI and GL account for available carbohydrates (sugars and starches) but not indigestible carbohydrates (fiber), as fiber passes through the gastrointestinal tract without being absorbed, thereby not directly affecting blood glucose levels. Hence, in calculating a food’s GL, fiber must be subtracted from total carbohydrates. Examples of GI and GL values for some common foods are presented in Table 1.
Table 1. Examples of GI and GL of Food Servings
| Food | Serving Size | Glycemic Index | Glycemic Load |
| Corn | 1 cup | 55 | 61.5 |
| Bagel | 1 bagel | 72 | 33 |
| Cola | 12 oz can | 63 | 25.2 |
| Donut | 1 donut | 76 | 24 |
| Lentils | 1 cup | 29 | 7 |
| Peach | 1 medium peach | 28 | 2.2 |
| Broccoli | 1 cup | 0 | 0 |
| Almonds | ¼ cup | 0 | 0 |
| Chicken | 3 oz | 0 | 0 |
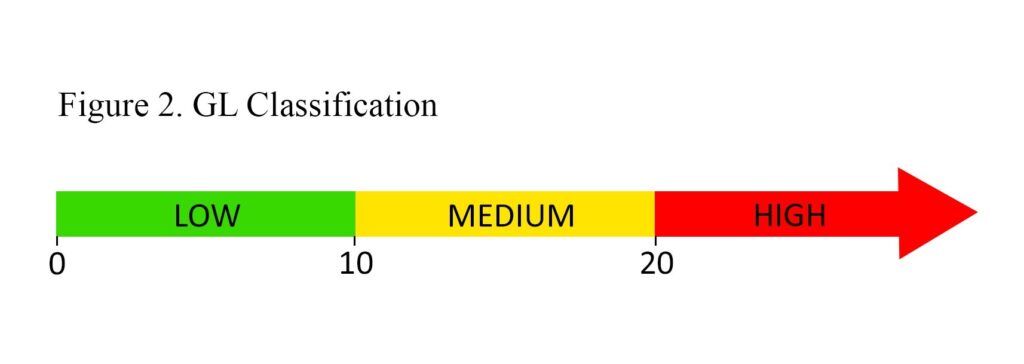
Classification of an individual food as having a low, medium, or high GL is based on ranges presented in Figure 2. Unfortunately, GL ranges to classify overall daily intake as being a low- or high-GL diet have not been universally established, possibly due to the variability in carbohydrate requirements of different populations (men vs women, young vs elderly, athletic vs sedentary). In comparing GL values used in research, a meta-analysis of 14 studies examining the effect of GL on obesity reported variable values used to classify low/high daily GL, but the addition of these values resulted in a GL cut-off of 95 (low daily GL <95; high daily GL ≥95).11 While this cut-off has not been confirmed as being clinically useful, it can be used in practice as a reference point.
 GL makes it possible for individuals to help manage blood glucose at the gut level. Low-GL foods contain complex carbohydrates and/or a low amount of available carbohydrates. Complex carbohydrates take longer to digest, resulting in a gradual rise in blood glucose and subsequent gradual rise in insulin. Experiencing chronic insulin spikes can lead to insulin resistance, which is associated with various negative health conditions, such as type 2 diabetes, obesity, cardiovascular disease, and cognitive impairment.12-14 Managing blood glucose is therefore extremely important for vitality and overall health, and low-GL diets can be utilized to help regulate glycemia.
GL makes it possible for individuals to help manage blood glucose at the gut level. Low-GL foods contain complex carbohydrates and/or a low amount of available carbohydrates. Complex carbohydrates take longer to digest, resulting in a gradual rise in blood glucose and subsequent gradual rise in insulin. Experiencing chronic insulin spikes can lead to insulin resistance, which is associated with various negative health conditions, such as type 2 diabetes, obesity, cardiovascular disease, and cognitive impairment.12-14 Managing blood glucose is therefore extremely important for vitality and overall health, and low-GL diets can be utilized to help regulate glycemia.
Glycemic Load & Mental Health
Preliminary evidence suggests that GL may be relevant in the pathogenesis and treatment of mental illnesses such as anxiety and depression. A multinational observational study comparing population sugar consumption and depression prevalence over a decade found a highly significant correlation,15 and a recent animal study that fed mice a high refined-carbohydrate diet for 12 weeks observed an increase in anxiety and depression behaviors.16 Several observational studies conducted in patients with diabetes show relationships between anxiety and depression and poorer glycemic control, although the direction of causality is unclear.17,18 A recent cohort study showed that increasing odds of depression and anxiety have been associated with the consumption of foods that have a progressively higher GI, a relationship that was maintained after controlling for micronutrients known to play a role in mental health.19 Cross-sectional studies have demonstrated a correlation between the occurrence of depression/stress and a higher intake of sweet foods, and a large prospective cohort study showed a positive correlation between depression risk and a diet high in sweet desserts and refined grains.20 Conversely, it was also found in this study that high consumption of fruits and vegetables, which contain fiber that lowers GI, was associated with a lower risk for depression.20
In addition to the observational studies, a limited number of interventional studies have explored a potential relationship between blood sugar balance and emotional and cognitive health. One clinical trial assigned healthy overweight subjects to high- or low-GI diets, and found that the high-GI diet resulted in worsening mood scores.21 Two studies assessed the impact of high- and low-GI meals on cognitive performance in adults with type 2 diabetes and children, respectively; both found that the higher-GI meal was related to poorer cognitive function.22,23 Conversely, 2 other intervention studies comparing high- and low-GI diets in patients with diabetes did not demonstrate changes in subclinical depression or cognitive function.20 Despite this evidence that a relationship between dietary GI and emotional and cognitive functioning may exist, no interventional studies have assessed the impact of a low-GI or low-GL dietary intervention in psychiatric patient populations in order to assess causality of the relationship. The following case from author MA’s clinical practice documents a case of anxiety in combination with hypoglycemia symptoms that responded to dietary modification. This case may shed light on the role of dietary glycemic load in the pathogenesis and treatment of mental illness.
Case Presentation
AB is a 15-year-old female student of South-Asian descent. She presented with concerns of anxiety and symptoms of hypoglycemia, as well as difficulty concentrating, fatigue, headaches, and frequent urinary and vaginal infections.
Her anxiety met the criteria for generalized anxiety disorder, and she rated the intensity of anxiety as 8/10, (10 = highest anxiety possible). The anxiety started 3 months prior to the initial appointment and had worsened in the previous month. She described excessive worry that was difficult to control and which impacted her daily functioning. She also experienced somatic symptoms, including heart palpitations, shakiness, discomfort in her stomach, and muscle tension. In response to the anxiety symptoms, she would eat foods like chocolate, chips, or fruit. AB was working with a counselor to manage the anxiety symptoms and was finding some benefit.
AB had experienced episodes suggestive of hypoglycemia since 12 years of age. The symptoms included muscle weakness and shaking, headaches, nausea, anxiety, and loss of concentration. Her symptoms were ameliorated by eating sweet foods.
Clinical Findings
A diet history revealed the following typical daily food intake:
- Breakfast: fruit smoothie containing fruit, fruit juice, and water
- Morning snack: bagel with margarine
- Lunch: pasta or white rice with vegetables
- Afternoon snack: granola bar or cookies or gummy candies
- After-school meal: white pasta, which may include meat
- Dinner: white rice or spaghetti, which may include meat
- Evening snack: cookies, toast
Past Medical History & Clinical Assessment
Due to her difficulty concentrating, AB had been prescribed dextroamphetamine, 5 mg. Repeat assessments of random and fasting blood glucose, as well as screening physical examination, were within normal range.
Therapeutic Approach
At the initial visit, the following dietary plan was prescribed:
- Breakfast: smoothie containing fruit, water, 1 scoop of protein powder, and 1 tablespoon of flax seeds or olive oil
- Lunch and dinner: include a serving of protein (meat, legume, soy) and a serving of a vegetables
- Snacks: include protein in snacks whenever possible (examples: apple with sunflower seed butter, vegetable sticks with hummus, pumpkin seeds)
- Continue previous snacks as needed for hypoglycemia symptoms
While eggs, nuts, and fish would have normally been recommended as well, the patient had an anaphylactic allergy to these foods.
Follow-up & Outcomes
At the first follow-up, 4 weeks later, AB reported that she had complied with the dietary plan. She reported a significant decrease in anxiety (4 to 5/10), as well as improved energy, less frequent and less intense hypoglycemia symptoms, and fewer headaches, in addition to improved concentration and mood. She required fewer snacks during the day and had decreased her intake of granola bars, cookies, and candies (1-2 servings per day). She also reported a cessation of chronic vaginal discharge. The substantial improvement in her anxiety symptoms prompted AB to temporarily discontinue her counseling sessions.
At a subsequent follow-up visit 4 weeks later, AB reported that she had briefly reverted back to her original diet for a period of 1 week and experienced a worsening of anxiety symptoms within 1 day. After returning to the dietary intervention prescribed, her anxiety symptoms decreased within 2 days.
Mechanism of GL’s Impact on Brain Health
One potential mechanism for the impact of dietary sugar on mental health is the triggering of reactive hypoglycemia. Ingestion of high-GI foods results in an increase in blood glucose levels; however, a large compensatory insulin release can result in reactive hypoglycemia, which is associated with an acute increase in epinephrine.24 This in turn contributes to neuropsychiatric symptoms including anxiety and symptoms associated with anxiety, such as shakiness, sweating, and heart palpitations.25 Induction of hypoglycemia in a laboratory setting has been shown to have a negative impact on mood, hedonic tone, and energy levels, and to produce an increase in tense arousal.26 The presence of both anxiety and hypoglycemia symptoms in the clinical case discussed in this article, along with their concurrent response to treatment, lends support to the hypothesis that these conditions may be related.
Another potential mechanism for the link between GL and mental health is increased levels of oxidative stress. Elevated blood glucose levels promote oxidative stress by increasing the generation of reactive oxygen species, reactive nitrogen species, lipid peroxidation, protein oxidation, and decreasing antioxidant levels.27 In addition, hyperglycemia activates the diacylglycerol protein kinase C pathway and increases production of advanced glycation end-products, both of which promote oxidative stress.28 An increase in oxidative stress negatively affects mental health by promoting neuronal death, either directly or through generating neuroinflammation.29 Oxidative stress and inflammation have been implicated in the development of depression, and higher levels of inflammatory and oxidative stress markers have been observed in depressed populations.30 In animals, increased levels of inflammation leads to symptoms of depression including malaise, weakness, listlessness, poor concentration, lethargy, decreased interested, and decreased appetite.31 Administering anti-inflammatory molecules to these experimental animals blocks these effects.32 In humans, studies have found that increasing levels of inflammation can lead to increased anxiety, irritability, hyper-arousal, and mania symptoms.32
Hyperglycemia is also strongly correlated with reduced hippocampal volumes,33 which is seen in depression34 and could be linked to neuronal death. Hippocampal neurogenesis is mediated by brain-derived neurotrophic factor (BDNF) and associated with mood regulation.30 A reduction in hippocampal BDNF levels has been shown to be induced by a high refined-sugar diet in rats, which was also associated with reduced neuronal plasticity.35
Clinical Application
Based on this preliminary evidence, as well as the well-established link between GL and physical health conditions, modification of dietary GL in patients with mental health concerns should be considered as part of a naturopathic treatment plan. Below are some practical strategies for patients to use.
Strategies for Decreasing the GL of a Meal
- Choose whole grains instead of refined grains (eg, brown rice, whole-grain pasta, whole-grain bread, quinoa instead of white rice, white pasta or white bread)
- Increase fiber content of a meal by including vegetables, pulses (eg, lentils, beans, chickpeas), and seeds
- Limit intake of foods with added sugar
References:
- Sarris J, Logan AC, Akbaraly TN, et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
- Lai JS, Hiles S, Bisquera A, et al. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014;99(1):181-197.
- Jacka FN, O’Neil A, Opie R, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15(1):23.
- Barragán-Rodriguez L, Rodriguez-Morán M, Guerrero-Romero F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res. 2008;21(4):218-223.
- Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9(5):e96905.
- Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25(9):713-719.
- Aucoin M, Bhardwaj S. Generalized Anxiety Disorder and Hypoglycemia Symptoms Improved with Diet Modification. Case Rep Psychiatry. 2016;2016:7165425.
- Garber A, Csizmadi I, Friedenreich CM, et al. Association between glycemic load and cognitive function in community-dwelling older adults: Results from the Brain in Motion study. Clin Nutr. 2017 Jul 17. pii: S0261-5614(17)30250-9. [Epub ahead of print]
- Selhub EM, Logan AC, Bested AC. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol. 2014;33:2.
- Augustin LS, Kendall CW, Jenkins DJ, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25(9):795-815.
- Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23(8):699-706.
- Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293-302.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473-481.
- Saedi E, Gheini MR, Faiz F, Arami MA. Diabetes mellitus and cognitive impairments. World J Diabetes. 2016;7(17):412-422.
- Westover AN, Marangell LB. A cross-national relationship between sugar consumption and major depression? Depress Anxiety. 2002;16(3):118-120.
- Santos CJ, Ferreira AVM, Oliveira AL, et al. Carbohydrate-enriched diet predispose to anxiety and depression-like behavior after stress in mice. Nutr Neurosci. 201;21(1):33-39.
- Crispin-Trebejo B, Robles-Cuadros MC, Bernabé-Ortiz A. Association between depression and glycemic control among type 2 diabetes patients in Lima, Peru. Asia Pac Psychiatry. 2015;7(4):419-426.
- Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: association with blood glucose monitoring and glycemic control. J Pediatr Psychol. 2010;35(4):415-425.
- Gangwisch JE, Hale L, Garcia L, et al. High glycemic index diet as a risk factor for depression: analyses from the Women’s Health Initiative. Am J Clin Nutr. 2015;102(2):454-463.
- Haghighatdoost F, Azadbakht L, Keshteli AH, et al. Glycemic index, glycemic load, and common psychological disorders. Am J Clin Nutr. 2016;103(1):201-209.
- Cheatham RA, Roberts SB, Das SK, et al. Long-term effects of provided low and high glycemic load low energy diets on mood and cognition. Physiol Behav. 2009;98(3):374-379.
- Ingwersen J, Defeyter MA, Kennedy DO, et al. A low glycaemic index breakfast cereal preferentially prevents children’s cognitive performance from declining throughout the morning. Appetite. 2007;49(1):240-244.
- Papanikolaou Y, Palmer H, Binns MA, et al. Better cognitive performance following a low-glycaemic-index compared with a high-glycaemic-index carbohydrate meal in adults with type 2 diabetes. Diabetologia. 2006;49(5):855-862.
- Sejling AS, Kjaer TW, Pedersen-Bjergaard U, et al. Hypoglycemia-associated changes in the electroencephalogram in patients with type 1 diabetes and normal hypoglycemia awareness or unawareness. Diabetes. 2015;64(5):1760-1769.
- Paine NJ, Watkins LL, Blumenthal JA, et al. Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure. Psychosom Med. 2015;77(2):136-144.
- Gold AE, MacLeod KM, Frier BM, Deary IJ. Changes in mood during acute hypoglycemia in healthy participants. J Pers Soc Psychol. 1995;68(3):498-504.
- Xu X, Guo L,Tian G. Diabetes cognitive impairments and the effect of traditional chinese herbs. Evid Based Complement Alternat Med. 2013;2013:649396.
- Sena CM, Pereira AM, Seiça R. Endothelial dysfunction – a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832(12):2216-2231.
- Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology. 2015 Jan 10. doi: 10.1111/imm.12443. [Epub ahead of print]
- Marx W, Moseley G, Berk M, Jacka F. Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. 2017;76(4):427-436.
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):760-768.
- Müller N, Myint AM, Schwarz MJ. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders–relation to drug treatment. Dialogues Clin Neurosci. 2009;11(3):319-332.
- Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol Aging. 2011;32(11):1942-1948.
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(1):41-54.
- Molteni R, Barnard RJ, Ying Z, et al. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112(4):803-814.
Image Copyright: <a href=’https://www.123rf.com/profile_endomedion’>endomedion / 123RF Stock Photo</a>
 Anna Garber, MSc, is a student at the Canadian College of Naturopathic Medicine and a graduate of the University of Calgary and University of Toronto. Her MSc work explored the effect of glycemic load on cognitive function in older adults and was published in Clinical Nutrition. Anna is passionate about the role of diet in health, and one of her hobbies includes managing a recipe blog (www.kleankuisine.com).
Anna Garber, MSc, is a student at the Canadian College of Naturopathic Medicine and a graduate of the University of Calgary and University of Toronto. Her MSc work explored the effect of glycemic load on cognitive function in older adults and was published in Clinical Nutrition. Anna is passionate about the role of diet in health, and one of her hobbies includes managing a recipe blog (www.kleankuisine.com).
 Monique Aucoin, ND, is a naturopathic doctor and research fellow at the Canadian College of Naturopathic Medicine. Dr Aucoin’s clinical and research interests are focused on the role of diet in the treatment and prevention of mental illness. She has been involved in a range of systematic reviews and RCTs using natural health products, and she is passionate about supporting naturopathic doctors and students in engaging with evidence. For more info: www.MoniqueAucoinND.com
Monique Aucoin, ND, is a naturopathic doctor and research fellow at the Canadian College of Naturopathic Medicine. Dr Aucoin’s clinical and research interests are focused on the role of diet in the treatment and prevention of mental illness. She has been involved in a range of systematic reviews and RCTs using natural health products, and she is passionate about supporting naturopathic doctors and students in engaging with evidence. For more info: www.MoniqueAucoinND.com
 Sukriti Bhardwaj, BHSc, is a 3rd-year student at CCNM and is currently working as a research assistant within the Department of Research and Clinical Epidemiology. Sukriti has a keen interest in integrative oncology and the role that naturopathic therapies can play in the health and well-being of cancer patients. In addition to her interest in oncology, she has co-authored papers within the fields of psychiatry and digestive health. She appreciates that research has the potential to improve patients’ lives on a large scale, and is dedicated to contributing to the constantly growing body of literature in naturopathic medicine.
Sukriti Bhardwaj, BHSc, is a 3rd-year student at CCNM and is currently working as a research assistant within the Department of Research and Clinical Epidemiology. Sukriti has a keen interest in integrative oncology and the role that naturopathic therapies can play in the health and well-being of cancer patients. In addition to her interest in oncology, she has co-authored papers within the fields of psychiatry and digestive health. She appreciates that research has the potential to improve patients’ lives on a large scale, and is dedicated to contributing to the constantly growing body of literature in naturopathic medicine.