Setareh Tais, ND
In the seminal fluid, as secreted by the testicles, a substance or several substances exist which, entering the blood by resorption, have a most-essential use in giving strength to the nervous system and to other parts. But if what may be called spermatic anaemia leads to that conclusion, the opposite state, which can be named spermatic plethora, gives as strong a testimony in favour of that conclusion. It is known that well-organized men, especially from twenty to thirty-five years of age, who remain absolutely free from sexual intercourse or any other causes of expenditure of seminal fluid, are in a state of excitement, giving them a great, although abnormal, physical and mental activity.
Dr Charles-Edouard Brown-Séquard,
in an 1889 letter to The Lancet1
Introduction
Infertility, or failure for a couple to conceive after 12 months of unprotected intercourse, affects 15% of all couples, and almost 50% of these cases are attributed to male factor infertility. Evidence suggests that male fertility has been diminishing in the past several decades, with an estimated decline in the mean sperm count of approximately 0.94 × 106/mL per year.2 Hormonal imbalances, oxidative stress, endocrine disruptors, urogenital abnormalities, and poor lifestyle choices have commonly been blamed for infertility in men. Of note,hypogonadism is fairly common in the middle-aged and older male population and is strongly associated with various chronic conditions such as type 2 diabetes mellitus, metabolic syndrome, obesity, hypertension, and osteoporosis. Therefore, primary care physicians are increasingly likely to encounter this population and engage in discussions on treating hypogonadism. The Endocrine Society3 defines hypogonadism in men as a clinical syndrome that results from failure of the testes to produce adequate levels of testosterone and a normal number of sperm due to a disruption of the hypothalamic-pituitary-gonadal (HPG) axis. It is diagnosed by a low sperm count or a low serum testosterone level, as well as the presence of hypogonadal symptoms such as a reduction in fertility, muscle mass, peak bone mass, energy, erectile function, mood, and libido.
Production of testosterone is regulated by the HPG axis, whereby the hypothalamus produces gonadotropin-releasing hormone (GnRH), which stimulates the anterior pituitary to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH). The FSH stimulates spermatogenesis by acting on the Sertoli cells, while the LH stimulates the Leydig cells of the testes to secrete testosterone. Testosterone levels modulate the HPG axis, such that high testosterone levels inhibit the frequency and amplitude of the GnRH pulsatile release from the hypothalamus. This testosterone-medicated inhibition reduces FSH- and LH-mediated stimulation of the Leydig and Sertoli cells.4-6 Although testosterone has been shown to have an important role in spermatogenesis,7 administration of exogenous testosterone has a dramatic inhibitory effect on spermatogenesis by feedback inhibition of the HPG axis. Therefore, while exogenous testosterone therapy is an effective method for increasing testosterone levels, it is not a viable option for men who desire an improvement in their fertility. In fact, a prospective multicenter contraceptive efficacy study8 in 1996 found that weekly intramuscular injections of testosterone enanthate (200 mg) reduced sperm counts to severe oligospermia or azoospermia. Similar results were seen with subdermal implants of testosterone depots9,10 and with topical testosterone gel.11,12
Reproductive endocrinologists treat men having hypogonadism due to defects in the HPG axis with injectable medications such as recombinant FSH, human chorionic gonadotropin, human menopausal gonadotropin hormone, or GnRH. Clomiphene citrate, an estrogen receptor antagonist, is an oral medication used to stimulate gonadotropin release from the pituitary. Several shortcomings exist, however, with all of these allopathic therapies: human chorionic gonadotropin can take up to 24 months to improve sperm count, human menopausal gonadotropin hormone is expensive, pulsatile GnRH is not widely available, and clomiphene citrate cannot be used in men with pituitary disease.13 Given these limitations, NDs are well positioned to offer their patients a more holistic approach to improving testosterone levels and fertility. Using herbal medicines and addressing the determinants of male reproductive health, naturopathic medicine is a wonderfully effective approach to improving hypogonadism in men who desire improved fertility.
Herbal Medicines for Improving Testosterone Level and Sperm Count
Ashwagandha, also known as Indian ginseng or Withania somnifera, has been described in Ayurvedic medicine as an aphrodisiac, used to treat male sexual dysfunction and infertility.14-16 Recently, a prospective study17 was conducted to assess the effect of ashwagandha root on semen variables, oxidative biomarkers, and hormone levels among infertile young men, aged 25 to 40 years, in India. The control group was composed of 75 men with proven fertility (history of achieving pregnancy) and normal results on semen analyses. Seventy-five men with infertility and abnormalities in their semen variables (defined by low sperm count, motility, and morphology) were given ashwagandha root (5 g/d) with milk for 3 months. Semen analyses, seminal plasma, and venous blood samples were obtained in all men at baseline and at 3 months. The results demonstrated that ashwagandha root increased testosterone and LH, while decreasing FSH and prolactin, among infertile men having suboptimal testosterone levels compared with the controls. Complementing these findings, ashwagandha treatment resulted in a significant increase in sperm count, concentration, and motility among infertile men. Not surprising, infertile men treated with ashwagandha also demonstrated an improvement in reduced glutathione and superoxide dismutase levels.17
Another herbal medicine to be considered for treating hypogonadism in infertile men is Mucuna pruriens, known commonly as velvet bean and cowitch, which is also described in Ayurvedic medicine as an aphrodisiac that can help improve sperm count.18 Another prospective study19 was conducted to assess the effect of the seeds of M pruriens on semen variables among infertile young men, aged 30 to 40 years, in India. The control group was composed of sixty men with proven fertility and normal results on semen analyses. Sixty men with infertility and an abnormal semen analysis (defined by low sperm count, motility, and morphology) were given Mucuna seed (5 g/d) with milk for 3 months. Semen analyses and seminal plasma samples were obtained in all men at baseline and at 3 months. The results demonstrated that treatment with Mucuna improved sperm count and motility in infertile men compared with the control group. The authors19 proposed that M pruriens, a rich source of l-dopa,20 stimulates the hypothalamus to secrete GnRH. A similar prospective study by Shukla et al21 corroborated these findings by demonstrating that treatment with Mucuna seed significantly improved testosterone levels and semen variables.
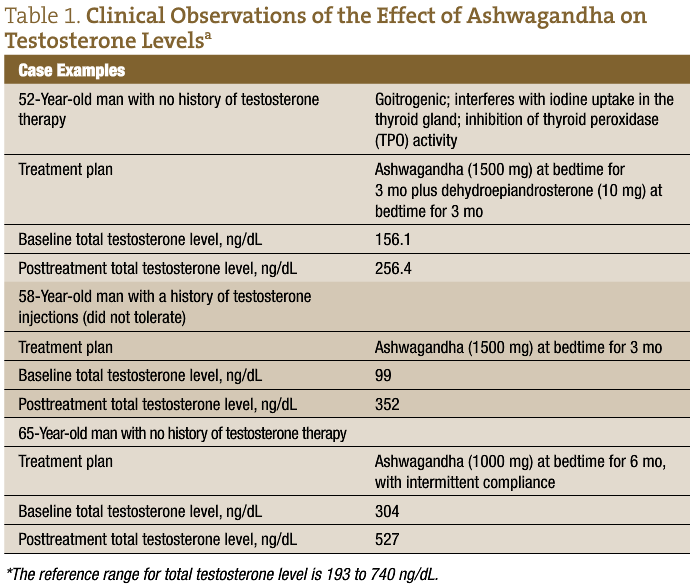
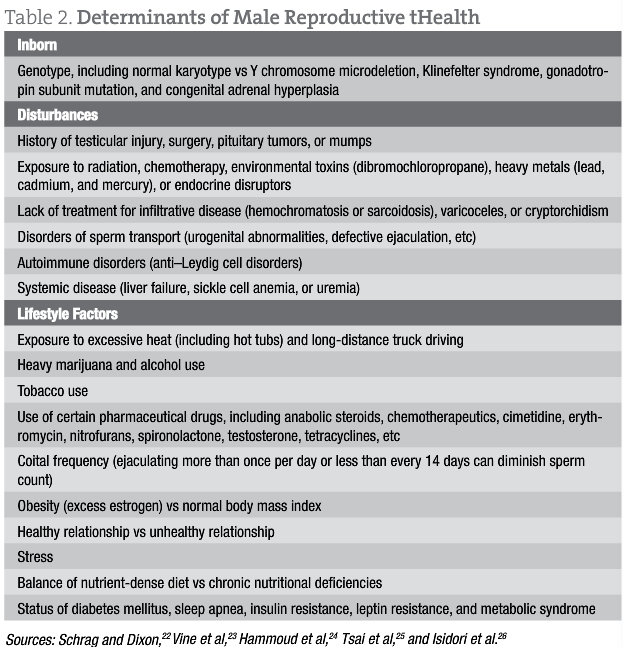
These findings suggest that W somnifera and M pruriens are effective for restoring sex hormones in infertile men and male reproductive health (Table 1 and Table 2). Given the prevalence of hypogonadism, the desire for improved fertility among those having difficulty conceiving, and the shortcomings of allopathic treatments, a naturopathic approach to treating low testosterone levels with concomitant infertility is a promising and worthwhile option to offer patients who are seeking a more holistic approach to male reproductive health.
Acknowledgment: I would like to express my appreciation to Kasra Pournadeali, ND, for his feedback and insight.
Setareh Tais, ND practices family medicine with a focus on women’s health, pediatrics, and reproductive health. She received her doctorate of naturopathic medicine from Bastyr University (Kenmore, Washington) and completed a 2-year family medicine residency at a Bastyr affiliate site. During her residency, she received additional training in reproductive endocrinology and infertility at Pacific Northwest Fertility (Seattle, Washington) and at Seattle Reproductive Medicine.
References
Brown-Séquard CE. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet. 1889;3438:105-107.
Carlsen EL, Giwercmn A, Keiding N. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609-613.
Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline [published correction appears in J Clin Endocrinol Metab. 2006;91(7):2688]. J Clin Endocrinol Metab. 2006;91(6):1995-2010.
Bagatell CJ, Bremner WJ. Androgens in men: uses and abuses. N Engl J Med. 1996;334:707-714.
Costanzo LS. Physiology. 3rd ed. Philadelphia, PA: Saunders Elsevier; 2006.
Dandona P, Rosenberg MT. A practical guide to male hypogonadism in the primary care setting. Int J Clin Pract. 2010;64(6):682-696.
Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397-406.
World Health Organization. Contraceptive efficacy of testosterone-induced azoospermia and oligospermia in normal men [published correction appears in Fertil Steril. 1996;65(6):1267]. Fertil Steril. 1996;65(4):821-829.
Handelsman DJ, Conway AJ, Boylan LM. Suppression of human spermatogenesis by testosterone implants. J Clin Endocrinol Metab. 1992;75(5):1326-1332.
Handelsman DJ, Conway AJ, Howe CJ, Turner L, Mackey MA. Establishing the minimum effective dose and additive effects of depot progestin in pressure of human spermatogenesis by a testosterone depot. J Clin Endocrinol Metab. 1996;81(11):4113-4121.
Mahabadi V, Amory JK, Swerdloff RS, et al. Combined transdermal testosterone gel and the progestin nestorone suppresses serum gonadotropins in men. J Clin Endocrinol Metab. 2009;94(7):2313-2320.
Soufir JC, Meduri G, Ziyyat A. Spermatogenetic inhibition in men taking a combination of oral medroxyprogesterone acetate and percutaneous testosterone as a male contraceptive method. Hum Reprod. 2011;26(7):1708-1714.
Büchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998;139(3):298-303.
Nandkarni KM. Indian Materia Medica. Bombay, India: Popular Prakashan; 1986:153-155.
Misra LC, Singh BB, Degenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5(4):334-346.
Abdel-Magief EM, Abdel-Rehman HA, Harraz FM. Effects of extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. J Ethnopharmacol. 2000;75(1):1-4.
Ahmad MK, Mahdi AA, Shukla KK, et al. Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile men. Fertil Steril. 2010;94(3):989-996.
Kumar KVA, Srinivasan KK, Shanbhag T, Rao SG. Aphrodisiac activity of the seeds of Mucuna pruriens. Indian Drug. 1994;31:321-327.
Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Effects of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90:627-635.
Prakash D, Niranjan A, Tewari SK. Some nutritional properties of the seeds of three Mucuna species. Int J Food Sci Nutr. 2001;52(1):79-82.
Shukla KK, Mahdi AA, Ahmad MK, Shankhwar SN, Rajender S, Jaiswar SP. Mucuna pruriens improves male infertility by its action on the hypothalamic-pituitary-gonadal axis. Fertil Steril. 2009;92(6):1934-1940.
Schrag SD, Dixon RL. Occupational exposures associated with male reproductive dysfunction. Annu Rev Pharmacol Toxicol. 1985;25:567-592.
Vine MF, Margolin BH, Morrison HI, Hulka BS. Cigarette smoking and sperm density: a meta-analysis. Fertil Steril. 1994;61(1):35-43.
Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT. Obesity and male reproductive potential. J Androl. 2006;27(5):619-626.
Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone–binding globulin and body far. Diabetes Care. 2004;27(4):861-868.
Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84(10):3673-3680-3680.


