SERENA RUSSUM
TERRANCE MANNING II, ND, RMSK
A 44-year-old female presented via telemedicine with new-onset right shoulder pain. Her pain followed no obvious inciting event, and it had progressively worsened over 5 months. The pain began after she transitioned to working from home, increasing the amount of time she spent at her computer (8-10 hours per day). She described the pain as tight, sharp, and radiating from the shoulder into her neck and forearm. She denied any paresthesia or numbness. The patient’s pain was worse with movement, which limited her activities. At rest, the pain was minimal, but could reach a level of 8/10 (10 being the worst) with movement, and it was aggravated by sleeping on the ipsilateral side. She had attended 8 weeks of physical therapy (PT), with no improvement.
Patient Evaluation
Physical exam (PE) was performed via telehealth. The patient appeared in good general health but was obese (BMI of 60). The cervical spine was examined and did not have contributory findings. The right shoulder was negative for misalignment, erythema, or swelling. The right supraspinatus (SST), deltoid, and subscapularis at insertion points were all tender to palpation. Active range of motion (AROM) of the right shoulder was limited, internal rotation was 45°, abduction was 90° (with pain starting at 35°), and forward flexion was normal. O’Brien’s active compression test and the Empty Can Test were both positive on the right side. Hawkins-Kennedy test was negative.
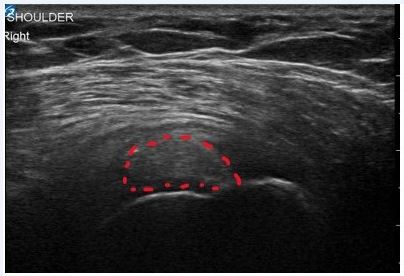
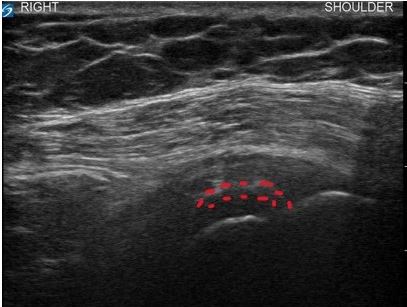
On a subsequent visit, diagnostic ultrasound (US) was performed on the patient’s right shoulder. US revealed a large calcification measuring (1.5 cm x 0.7 cm x 1.7 cm) within the SST, a thickening of the subdeltoid bursa, and mild synovitis of the acromioclavicular joint (Figure 1a). All other shoulder anatomy was unremarkable upon US visualization. Clinical and diagnostic US findings were consistent with subacute calcific tendinitis of the supraspinatus tendon as the etiology of patient’s symptoms. Given the subacute presentation and severity of symptoms, as well as the failure of other conservative measures, local treatment was warranted at this time.
Figure 1a. Pre-Treatment Findings
Etiopathogenesis
Shoulder calcific tendinitis (CT) is characterized by calcium salt depositions in the mid-substance or insertion of rotator cuff tendons and synovial tissues.1-5 The deposits consist of calcium carbonate hydroxyapatite, ranging from amorphous to semi-solid crystalline structures; suggesting variations in maturation that have been categorized into 3 stages, the second stage of which has 3 subphases1,2,4,5:
- Pre-calcific stage
- Calcific stage
- Formative phase
- Resting phase
- Resorptive phase
- Repair stage
CT is the most common cause of non-traumatic shoulder pain, but can be asymptomatic in 20-50% of cases.1,3-5 It is unclear why calcium develops; factors associated not only with symptom development but earlier onset and increased chronic pain include high BMI, female sex, older age, hypothyroidism, and hyperlipidemia.4,6-8 When symptomatic, patients will present with pain accompanying specific movements, limits in range of motion, aggravation of pain while sleeping, and local edema.1,3-5
The incidence of CT varies, affecting 2.7-22% of the general population and up to 30% of patients with insulin-dependent diabetes.1,4,5,7 CT most commonly affects 30-60-year-olds, with women affected 2 times more often than men.1,3,4,7,8 About 10-25% of cases are bilateral, and 80% of cases affect the SST.1,3-5 CT is not associated with manual work, but is very common in those who maintain internal rotation with slight abduction, such as desk workers.3-5,9 This corresponds to a hypovascular area of the SST.4 CT is often self-limiting, with spontaneous resolution occurring in 70% of cases within 4 years; however, fibrocartilaginous tissue can form around the crystalline structure, blocking immune cells and preventing resolution.3 Additional complications can also occur if left untreated.1,5,10
The most accepted etiopathogenesis theory (by Uhtoff) purposes CT as an active cell-mediated process featuring chondrocyte metaplasia in the area.2,3 It is assumed that these chondrocytes then mediate the progression of the disease by producing calcium deposits within the tendon matrix. Still, the initial trigger of these processes is poorly understood and is likely due to numerous factors.3 Some studies have shown tendon-derived stem cells differentiating into chondrocytes.3,4 This is possibly due to a failed cell-mediated healing response to repetitive microtrauma to specific areas of the tendon with poor vascularity.4 The calcium deposition process is poorly understood, but the presences of certain proteins, like osteopontin (a bone matrix protein associated with bone formation and mineralization) commonly found around sites of CT, may provide a scaffolding-type framework as evidence of the calcium deposit as a natural response of the body to heal a deficit within the tendon matrix. Thus, in a tendon that has a non-traumatic (eg, repetitive microtrauma) disruption of its collagen matrix in a poorly vascularized portion that shares a border with bone, the damaged area does not heal via neovascularization; rather, it absorbs calcium from the bone in order to repair the local matrix. Unfortunately, however, this attempt to heal the area results in a large inflammatory response, leading to exquisitely painful (in the acute phase) calcific tendinitis.
Therapeutic Intervention
The minimally invasive intervention of ultrasound-guided percutaneous single needle aspiration and lavage (UGPSN-A&L) with subdeltoid bursal corticosteroid injection was chosen as the initial therapy.1,4,11-13 This procedure is considered safe and is an effective non-operative treatment.1,4,11-13 It also has the advantage of being performed immediately in-office upon diagnosis. The procedure entails a slow and pulsatile injection of saline into the crystalline structure, without disrupting its borders, in order to soften the calcium and provide pressure that forces the return of the free calcium into the syringe as pressure on the plunger is released.4,12,13 This pulsatile flushing commences until adequate dissolution and reduction in calcium volume has been achieved. If effective, symptoms typically resolve within 3 months, and approximately 70% of patients achieve symptom resolution within 6 months.5,14
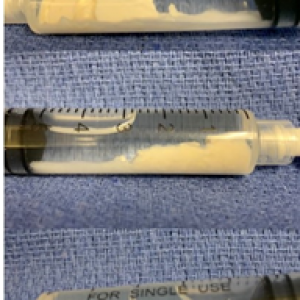
In this case, sterile technique was used throughout the procedure. Local anesthesia was achieved via 2% lidocaine buffered with sodium bicarbonate. An 18-gauge 3.5-inch needle was inserted into the calcium deposit under US-guidance. Images were obtained that demonstrated precise needle placement (Figure 1b). Three separate 5-mL syringes of sterile saline were used to dissolve and remove the calcium deposit (Figure 2). Under continual US-guidance, the needle was redirected into the subdeltoid bursa. A total of 5 mL of solution (containing 1 mL [40 mg] of methylprednisolone acetate, 2.5 mL of 10% dextrose, 1.5 mL of 0.5% ropivacaine HCl) was injected into the bursa. US images demonstrated accurate needle and injectate placement. The procedure was completed without complications and was tolerated well by the patient. She was instructed to use conservative therapies (ice, heat, acetaminophen) as needed to manage pain, to avoid strenuous activity for 24-48 hours, to call immediately with any signs of infection, to follow up in 2 weeks to monitor progress, and to start PT at that time.12,13
Figure 1b. Needle Placement
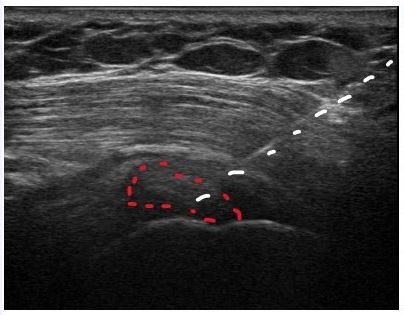
Figure 2. Aspirated Calcium
Follow-up and Outcomes
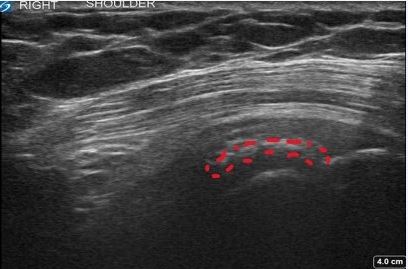
At the 2-week follow-up, the patient had started PT. It was recommended she continue the PT for at least 4 weeks. She reported significant improvement with full resolution of radiating symptoms; she was able to sleep comfortably and perform many activities without pain. PE revealed normal right shoulder AROM in all directions without pain. US showed persistent calcification but also indications of progress, including a lower volume of calcium and improved echotexture of previously identified calcification of the SST (Figure 1c). The patient’s progress was within expectations and signified a favorable prognosis for an excellent outcome. At this visit, there was in-depth discussion about diet and lifestyle modifications targeting underlying endocrine, metabolic, and hormonal contributions to the development of CT in this case and to prevent recurrence.1,4,5,7
Figure 1c. 2 Weeks Post-Injection
At 12 weeks, the patient reported no pain and overall significant satisfaction with the outcome of the procedure. PE was unremarkable. US revealed persistent remnant calcification, but improvement was obvious upon comparison with the previous US. Not only was the volume of calcium greatly reduced, but the secondary inflammatory change (tendinitis and subdeltoid bursitis) were also resolved (Figure 1d). Given the continually improved images and the absence of symptoms, no further local intervention was warranted. However, the patient was instructed to continue with diet and lifestyle changes to address overall health. Furthermore, she was referred to another naturopathic provider for hormonal optimization therapy. Ultimately, serial follow-up will commence every 4 weeks until ultrasound findings show complete resolution of calcium, which is the expected long-term outcome.
Figure 1d. 12 Weeks Post-Injection
Discussion
Calcific tendinitis is an often excruciatingly painful condition that is commonly encountered in the primary care setting but which can be difficult to quickly and adequately treat. Diagnosis requires imaging studies. Radiographs of the shoulder can identify radiopaque calcium deposits within the rotator cuff tendons, but X-ray may not be available at point of care. Furthermore, radiographs do not provide crucial information regarding the texture of the calcium or the associated inflammatory changes or tendinopathies. MRI can identify calcium deposits and associated inflammatory changes or tendinopathies; however, this technology does not indicate the texture of the calcium. In addition, calcium deposits can be easily missed on MRI, especially those with low volume. Lastly, MRI is expensive and requires insurance prior authorization, which can significantly delay care.
With point-of-care ultrasound gaining in popularity and the advent of affordable handheld transducers, this technology has many advantages to aid in both the diagnosis and treatment of rotator cuff calcific tendinitis. Ultrasound can identify calcium within a tendon (even those of small volume/punctate size), evaluate the texture of the calcium (eg, evaluate whether it shadows or not, is well encapsulated in a discrete pocket, or is multiloculated and diffuse within the tendon), and identify associated conditions. Furthermore, ultrasound can then be used to guide treatment of the condition, such as the ultrasound-guided percutaneous single needle aspiration and lavage technique described in this case.
Currently, there is debate on what therapies would best treat the various CT presentations.1,4,5,10,12,13 Regarding UGPSN-A&L, several studies question the long-term effectiveness and general validity of trials, given the lack of control groups.4,5,11-13,15 Few studies have looked at positive predictive factors regarding treatment outcomes.16-18
In light of the current popularity of regenerative injection therapies, this case provides some important lessons. Acute calcific tendinitis is not likely to respond to injection therapy as the initial treatment option. Regardless of choice of therapy (ie, prolotherapy, platelet-rich plasma, or cellular orthobiologics), if injection therapy is pursued in a case of calcific tendinitis without first aspirating the calcium, the injection therapy has a high likelihood of failure. The calcium must be removed to allow the remaining healthy tendon to approximate. If performing injection therapies, please be sure to perform diagnostic imaging before proceeding with therapy. If the root cause of a patient’s shoulder pain is calcific tendinitis yet you treated “shoulder pain” assuming hypermobility or tendinosis as the underlying cause, you could delay care and prolong the patient’s suffering. Conversely, once the calcium is successfully aspirated and the patient has modified their diet, lifestyle, and hormonal balance, then you might consider treating the involved tendon with regenerative injection therapy in hopes of accelerating recovery and healing the collagen matrix.4,19
References:
- Umamahesvaran B, Sambandam SN, Mounasamy V, et al. Calcifying Tendinitis of Shoulder: A Concise Review. J Orthop. 2018;15(3):776-782.
- Chiou HJ, Hung SC, Lin SY, et al. Correlations among mineral components, progressive calcification process and clinical symptoms of calcific tendonitis. Rheumatology (Oxford). 2010;49(3):548-555.
- Darrieutort-Laffite C, Blanchard F, Le Goff B. Calcific tendonitis of the rotator cuff: From formation to resorption. Joint Bone Spine. 2018;85(6):687-692.
- Sansone V, Maiorano E, Galluzzo A, Pascale V. Calcific tendinopathy of the shoulder: clinical perspectives into the mechanisms, pathogenesis, and treatment. Orthop Res Rev. 2018;10:63-72.
- Merolla G, Singh S, Paladini P, Porcellini G. Calcific tendinitis of the rotator cuff: state of the art in diagnosis and treatment. J Orthop Traumatol. 2016;17(1):7-14.
- Sansone V, Consonni O, Maiorano E, et al. Calcific tendinopathy of the rotator cuff: the correlation between pain and imaging features in symptomatic and asymptomatic female shoulders. Skeletal Radiol. 2016;45(1):49-55.
- Harvie P, Pollard TC, Carr AJ. Calcific tendinitis: natural history and association with endocrine disorders. J Shoulder Elbow Surg. 2007;16(2):169-173.
- Lin CC, Nfor ON, Su CL, et al. Interactive associations of sex and hyperlipidemia with calcific tendinitis of the shoulder in Taiwanese adults. Medicine (Baltimore). 2020;99(46):e23299.
- Sansone VC, Meroni R, Boria P, et al. Are occupational repetitive movements of the upper arm associated with rotator cuff calcific tendinopathies? Rheumatol Int. 2015;35(2):273-280.
- Merolla G, Bhat MG, Paladini P, Porcellini G. Complications of calcific tendinitis of the shoulder: a concise review. J Orthop Traumatol. 2015;16(3):175-183.
- Zhang T, Duan Y, Chen J, Chen X. Efficacy of ultrasound-guided percutaneous lavage for rotator cuff calcific tendinopathy: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98(21):e15552.
- Messina C, Sconfienza LM. Ultrasound-Guided Percutaneous Irrigation of Calcific Tendinopathy. Semin Musculoskelet Radiol. 2016;20(5):409-413.
- Pagnini F, D’Amuri FV, Bevilacqua A, et al. Ultrasound-guided percutaneous irrigation of calcific tendinopathy: technical developments. Acta Biomed. 2019;90(5-S):95-100.
- Yoo JC, Koh KH, Park WH, et al. The outcome of ultrasound-guided needle decompression and steroid injection in calcific tendinitis. J Shoulder Elbow Surg. 2010;19(4):596-600.
- Castillo-González FD, Ramos-Álvarez JJ, Rodríguez-Fabián G, et al. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2014;4(2):220-225.
- Oudelaar BW, Huis In ‘t Veld R, Schepers-Bok R, et al. Prognostic factors for the outcome of needle aspiration of calcific deposits for rotator cuff calcific tendinitis. Eur Radiol. 2020;30(7):4082-4090.
- Bazzocchi A, Pelotti P, Serraino S, et al. Ultrasound imaging-guided percutaneous treatment of rotator cuff calcific tendinitis: success in short-term outcome. Br J Radiol. 2016;89(1057):20150407.
- Erickson JL, Jagim AR. Ultrasonic Tenotomy and Debridement for Calcific Tendinopathy of the Shoulder: A Pilot Case Series. J Prim Care Community Health. 2020;11:2150132720964665.
- Gula G, Ketenci A. Treatment of calcific tendinitis of the rotator cuff with platelet-rich plasma injection: A case report. Agri. 2019;31(2):107-110.

Serena Russum is a 4th-year naturopathic student at the National University of Natural Medicine; she will graduate in June 2021. Ms Russum believes in treating the root cause of a condition using evidence-based medicine and the least invasive method possible, while holding space for patients’ preferences and values. Her goals are to optimize health for all, but she has a special interest in women’s health and complex chronic conditions, including chronic pain. Upon graduation and licensure, Ms Russum plans to seek additional training in physical modalities, including injection and intravenous therapies.

Terrance Manning II, ND, RMSK is an interventional orthopedic and orthobiologic medicine specialist focusing on non-surgical treatment of joint, tendon, and ligament issues. Dr Manning’s 3-year post-graduate CNME-accredited residency in regenerative medicine is unique among his peers. He is an expert in diagnostic and interventional musculoskeletal ultrasound. He is adjunct clinical faculty at NUNM and hosts an off-site shift for training students in this field. Dr Manning is a founding member and current vice president of the Naturopathic Orthopedic Medicine Academy (NOMA).

