Flavocoxid: A Botanical Extract Acts as a Balanced Inhibitor of Multiple Inflammatory Pathways
David M. Brady, ND, DC, CCN, DACBN
Introduction
There is great concern in the medical community over the excessive use of both non-steroidal anti-inflammatory drugs (NSAID) such as COX-2 inhibitors, and steroidal anti-inflammatory medications. There is also concern over the use of narcotic pain relievers, due to potentially serious side effects and dependency. In fact, patients generally use substantial doses of these prescription drugs on a chronic or ongoing episodic basis, thereby resulting in increased risk of long-term side effects, particularly among the elderly. There are emerging highly standardized and evidence-based natural agents that effectively modulate the same enzyme pathways as anti-inflammatory medications, but with much lower side-effect profiles. One such extract backed by strong supporting human-outcome studies, flavocoxid, will be discussed in this article with emphasis on its safety profile and balanced action across a multitude of inflammatory pathways.
Discussion
Integrative medicine clinicians from various professional backgrounds and fields, including NDs, are encountering an ever-growing population of patients/clients suffering from acute and chronic pain conditions, many of these being inflammatory in nature. Examples include sports injuries, degenerative and inflammatory arthritis, autoimmune-related disorders, and many more. Many of these patients experience significant gastrointestinal, renal, coagulation, and other side effects, and may not even be aware of them until they cause a serious medical disorder.1,2 Many of these same individuals would likely be interested in evidence-based, effective natural agents that reduce, and in many cases eliminate, the drug-associated side effects. Integrative providers need to understand the benefits and risks of standard interventions, as well as those of the available evidence-based complementary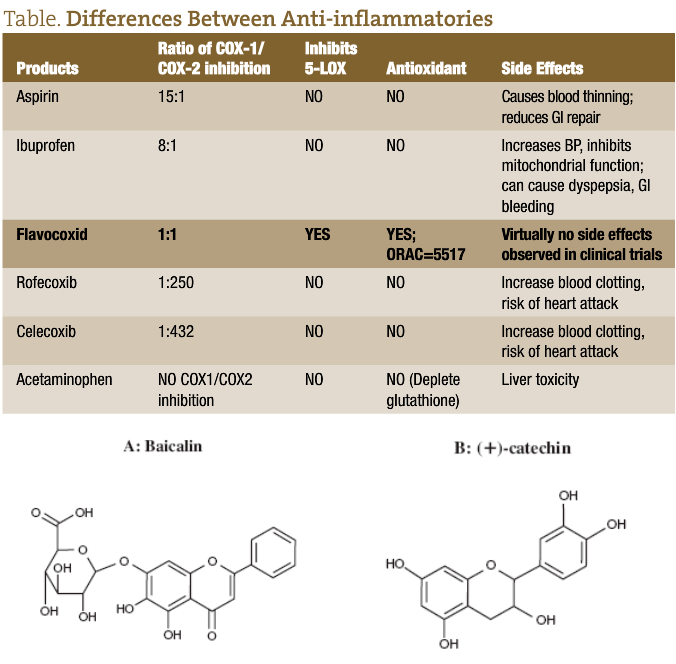 approaches.
approaches.
Some important facts about commonly used natural anti-inflammatory compounds3-84:
Natural anti-inflammatory compounds do not act as selective cyclooxygenase (COX) inhibitors. They constitute an array of compounds that have various combination effects of inhibiting both COX-2 and COX-1, in addition to the lipoxygenase (LOX) and phospholipase A2 enzymes. It is important that they also inhibit COX-1 to some degree because this provides a mild blood thinning effect, counteracting the blood clotting effect of COX-2 inhibition. In this sense, they act somewhat similar to non-selective NSAIDs (such as naproxen, diclofenac, and ibuprofen), although these medications do exert some dominance on the COX-1 isoenzyme. However, they also have many additional benefits, such as antioxidant activity, and do not promote gastrointestinal (GI) bleeding.
Natural anti-inflammatory agents may be a better choice for blood thinning than aspirin, which acts predominantly as a selective COX-1 inhibitor. Aspirin binds to platelets in an irreversible manner, resulting in serious risk of bleeding in cases of overdose and strong GI side effects. This is not the case with natural anti-inflammatory agents.
Natural anti-inflammatory compounds prevent the expression of “inducible” COX-2 which results from oxidative stress, due to the potent antioxidant effect of many of these compounds.
Proteolytic enzymes help reduce acute and chronic inflammation in ways unrelated to COX and LOX inhibition, such as the molecular debridement of the chemotaxis-promoting protein fragments and inflammatory mediators liberated from injured cells. These enzymes also have additional anti-thrombotic and anti-inflammatory effects.
Please refer to the table for a more complete understanding of the differences between the compounds referenced above.3
Flavocoxid
Flavocoxid is a specially manufactured and extensively studied natural food-based product composed of enriched plant extracts from two botanicals: Scutellaria baicalensis and Acacia catechu. Flavocoxid contains the same “active” constituents and micronutrients that can be found in many fruits, nuts, vegetables, and teas.85 Flavonoids, which are low-molecular weight compounds and part of the larger class of compounds known as polyphenols, are ubiquitous in plants.86 More than 9000 different flavonoids have been characterized, many of which are consumed regularly in the human diet. Their basic structure consists of a 3-ring nucleus with a large number of side chain possibilities.86 Flavocoxid contains 2 primary active ingredien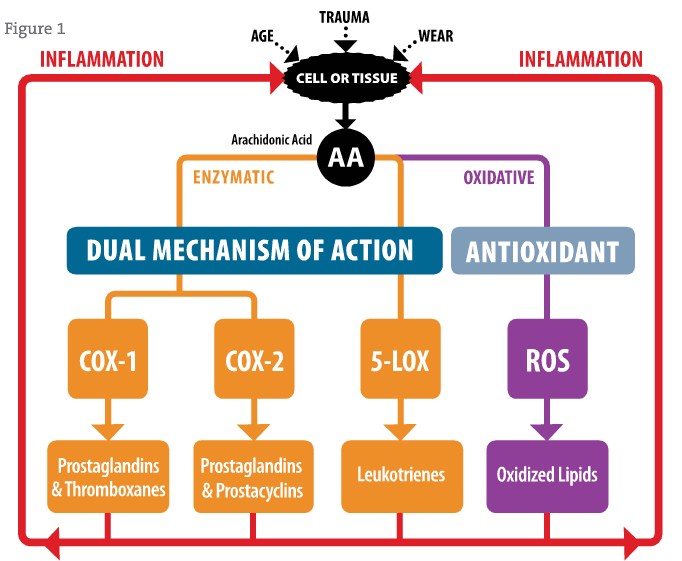 ts, baicalin and catechin. Baicalin is part of a larger class of flavonoids knows as free-B-ring flavonoids, whereas catechin is part of a class known as flavans. The plant sources for the free-B-ring, baicalin, and the flavan, catechin, have nutritional value and have been used in medicinal preparations and in foods for many years, especially in Asian countries.
ts, baicalin and catechin. Baicalin is part of a larger class of flavonoids knows as free-B-ring flavonoids, whereas catechin is part of a class known as flavans. The plant sources for the free-B-ring, baicalin, and the flavan, catechin, have nutritional value and have been used in medicinal preparations and in foods for many years, especially in Asian countries.
The development path for flavocoxid began in January of 1996 when a scientifically-based Korean ingredients supplier using pharmaceutical-like screening and development practices began to isolate multiple anti-inflammatory compounds from Aloe vera and Picrorhiza kurroa. This work was then followed by the development of high-throughput (HTP) extraction and fractionation methods of plant extracts in 1998. At the same time, screening of over 1200 plant extracts and tens of thousands of HTP fractions using the COX-1, COX-2, and 5-LOX enzymes began in earnest.87 Twenty-two initial plant extracts were identified having COX-1, COX-2, and 5-LOX activity. In secondary screening in cells, the company found that 14 of the initial 22 extracts were cytotoxic in monocytes. Further animal toxicity analysis showed that only 2 did not produce any significant changes in the physiology of animals, in terms of both acute and sub-chronic toxicity. These plant extracts from Scutellaria baicalensis and Acacia catechu were further characterized via HTP fractionation. It was further discovered that baicalin from Scutellaria baicalensis, and catechin from Acacia catechu, were the primary active ingredients in both plant fractionations having COX-1, COX-2, and 5-LOX activity.87
Extensive laboratory and animal toxicology testing followed in 2002-2003, characterizing the safety of the combination of these naturally derived compounds.88 This research showed that the molecules composing flavocoxid at certain ratios inhibited COX-1 and COX-2 enzymes to an equal degree while also inhibiting the 5-LOX enzyme (Figure 1). Results from these enzyme assays also correlated with observations made in cell assays. Animal models of inflammation also showed that flavocoxid could alleviate arachidonic acid-induced arthritis, considered part of the pathophysiology of osteoarthritis. In addition, later proteomic and genomic studies demonstrated that flavocoxid down-regulated specific cytokines involved in the initial inflammatory cascade after cell damage due to trauma, as well as COX-2 and 5-LOX production. These results suggest that flavocoxid has both protein and genomic inhibition effects for the management of inflammation. Drug interaction studies, as well as mutagenesis studies, have also been performed to assure safety of the extract.89
In 2002 through early 2003, human safety and efficacy testing ensued at Georgetown University, comparing placebo (n=39) versus flavocoxid (n=29) at 250 mg per day.88 No changes in blood electrolytes, serology, liver enzymes, renal markers, or general health were observed in this study. Any adverse events from flavocoxid were found to be comparable to placebo. Concurrently, initial efficacy testing was conducted at McGill University in Canada, comparing placebo (n=15), flavocoxid (n=15) at 125 mg BID, flavocoxid (n=15) at 250 mg BID, and celecoxib (n=15) at 100 mg BID. Significant reductions in pain and stiffness, as well as improvements in mobility, were observed for flavocoxid at both doses compared with celecoxib.90 These initial safety and efficacy studies were completed almost a year before marketing of flavocoxid as a medical food began in Puerto Rico in March of 2004.
In the GOAL study, a total of 1067 individuals at 41 rheumatology practices were enrolled and prescribed flavocoxid, 500 mg BID, for 60 days. The Physician Global Assessment of Disease (PGAD) visual analog scale (VAS) was used as a global measure to assess signs and symptoms of osteoarthritis (OA), including joint discomfort, functional stiffness, functional mobility and quality of life. In, addition, GI tolerability was assessed by individual questions scored on a 5-part Likert scale. Of the 1005 patients who completed all follow-up visits, physicians recorded an average improvement in VAS scores from 60.1 +18.8 at baseline to 42.5 +21.9 at 8 weeks (p<0.001) in 65.8% of patients, with the highest degree of improvement seen in those subjects with moderate-to-severe OA at baseline. An additional important finding was that, of those patients who had previously paused or interrupted their use of NSAID due to upper GI-related issues, ~90% tolerated flavocoxid and completed the study. The use of flavocoxid also resulted in a >30% reduction or cessation of the use of gastroprotective medications such as proton pump inhibitors (PPI) or histamine-2 receptor antagonists (H2s) in subjects.91
Post-Marketing Reported Adverse Events
Since the release of flavocoxid in 2004, there have been 4 reported cases of acute liver toxicity and elevated liver enzymes, which were attributed to individual reactions to flavocoxid. All subjects were women, ranging in age from 57 to 68 years. All developed symptoms and signs of liver injury within 1 to 3 months of starting flavocoxid and demonstrated elevated liver function tests (LFTs) and serum bilirubin. All serum markers returned to normal within 3 to 12 weeks after flavocoxid was discontinued, and all patients recovered without experiencing acute liver failure of chronic liver injury. Causality was adjudicated as highly likely in 3 patients and as possible in 1 patient.92
There have also been 7 confirmed reports of hypersensitivity pneumonitis.93 These events occurred sporadically and without warning or apparent predisposing factors. All required cessation of flavocoxid, as well as treatment with supplemental oxygen and parenteral corticosteroids. This is in contrast to the reduction in respiratory adverse events of an infectious nature seen in clinical trials of flavocoxid against placebo94 and against naproxen.95 These types of lung events are extremely rare but you should be aware of them in the unlikely event that you encounter one.
All therapeutic products that have significant physiological effects may have some level of potential toxicity and adverse effects, even a medical food with GRAS status like flavocoxid. Considering the hundreds of thousands of prescriptions filled for flavocoxid vs the number of reported significant side effects, flavocoxid maintains an impressive safety record when compared to other anti-inflammatory agents. However, it is recommended to monitor liver function tests on your flavocoxid patients according to the schedule you usually use in your practice, just as you would consider doing with other anti-inflammatory agents or medications. Post-marketing surveillance data on flavocoxid is collected on an ongoing basis to evaluate adverse events of patients while on flavocoxid. Currently, over 100 000 patients have been monitored by self-reporting or by physician-reported incidences, with an adverse event rate of ~0.1%. In a 2009 clinical trial,94 sponsored by an NIH grant (Phase I SBIR Grant #: 1 R41 AR051232-01) and conducted at the University of Alabama-Birmingham, no significant differences were found, in terms of adverse effects, between osteoarthritis patients treated with flavocoxid (250 mg BID; n=30) or placebo (n=29).
Genomic/Protein Mechanism of Action (MOA) Data
Flavocoxid reduced the protein expression of COX-2 and 5-LOX, though not COX-1, presumably through an antioxidant mechanism of action (MOA) in a macrophage cell model.96 This MOA was correlated to decreased NFκB activation and increased IκBα expression, the cytoplasmic control factor of NFκB. The expression of NFκB is controlled in part by oxidative activation. Tumor necrosis factor-α (TNFα) gene and protein expression, as well as iNOS protein expression, were also decreased through the same mechanism. Consequently, levels of PGE2, LTB4 and nitric oxide (NO) metabolites were decreased due to the corresponding protein down-regulation. Flavocoxid acted as an antioxidant, decreasing malondialdehyde (a direct oxidative product of arachidonic acid) concentrations in cell culture. This MOA data was confirmed in a Duchene muscular dystrophy (DMD) animal model by Messina et al.97
The importance of this antioxidant mechanism cannot be underestimated, especially for the management of chronic discomfort that occurs in osteoarthritis. All the molecules produced from the COX-2, 5-LOX and iNOS pathways (eg, prostaglandins, leukotrienes, and nitric oxide) bind to and activate the pain receptors, causing nociceptive pain signals to be transmitted back to the brain. In addition, cytokines also bind these receptors, as do a myriad reactive oxygen species (ROS). Flavocoxid, indicated for the clinical dietary management of osteoarthritis under physician supervision, works in all of these pathways and as a direct antioxidant on most of the ROS.
Head-to-Head Comparative Clinical Trials: Flavocoxid vs Naproxen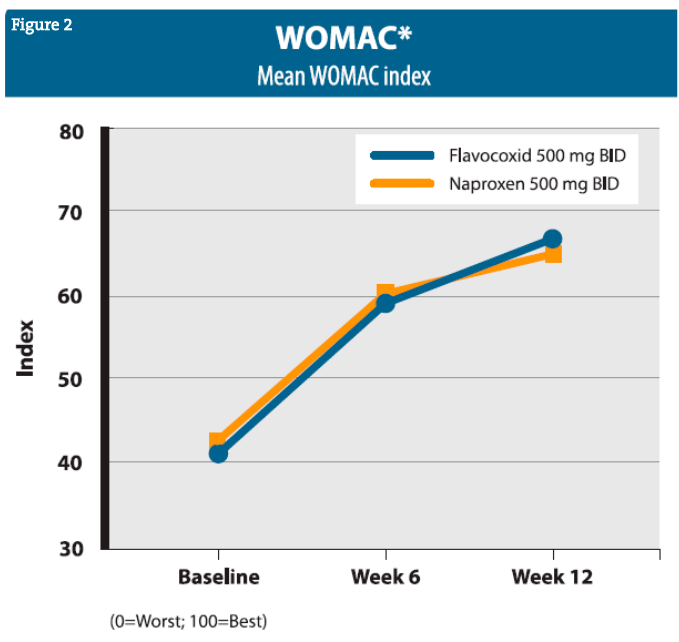
Clinical trials of flavocoxid have continued with a well-controlled, head-to-head comparative study of 103 patients with OA of the knee, treated with either flavocoxid (500 mg BID) or naproxen (500 mg BID) for 4 weeks.98 Flavocoxid and naproxen showed comparable effectiveness at alleviating OA signs and symptoms, and both appeared generally safe, though the subjects taking naproxen experienced more edema and musculoskeletal discomfort as side effects. In addition, further substantiation that flavocoxid does not interact with aspirin, or affect bleeding times or platelet aggregation was conducted and published by Pillai et al (99). A larger, well-controlled, head-to-head comparative study (n=220) of flavocoxid (500 mg BID) vs naproxen (500 mg BID) for 12 weeks was then published by Levy et al, showing comparative improvements in OA, as reflected by Western Ontario and McMasters Universities Osteoarthritis Index [WOMAC] scores (Figure 2).95 The flavocoxid group also showed comparatively fewer adverse effects. Further substantiation that flavocoxid does not interact with aspirin or affect bleeding times or platelet aggregation was provided and published by Pillai et al.99 Newer work on flavocoxid has included the study of its effect on collagen-induced arthritis in animal models100 and its effects in an animal model of induced pancreatitis.101
Commercial Availability
Flavocoxid is currently available in two commercially marketed medical food products designed to be used under the supervision of a qualified licensed health care provider: 1) a medical food that is available in 250-mg and 500-mg versions through conventional prescription, and 2) a professionally dispensed (via licensed health care provider practice dispensary or authorized patient-direct order from the manufacturer) medical food product that also contains novel functional collagen peptides for joint health.
The concept of a “medical food” is a relatively recent one, and this product category is growing rapidly. Products now regarded as medical foods were first regulated as drugs by the FDA until 1972, when the agency issued an Advance Notice of Proposed Rulemaking (ANPR), which it has since withdrawn. In 1988, Congress established the legal category of medical foods in the Orphan Drug Amendment, which states that “medical foods” are designed to be orally consumed, administered under the supervision of a physician, specially formulated and processed (ie, cannot be derived at the given concentration or formulation through a change in diet alone), and intended for the specific dietary management of a disease or condition that has distinctive nutritional requirements.102 That definition was subsequently incorporated into the Nutrition Labeling and Education Act of 1990 (NLEA), wherein Congress exempted medical foods from the nutrition labeling, health claim, and nutrient disclosure requirements applied to most other foods. This has created a territory somewhere between supplement and drug where products can be marketed with the supported medical claims as long as they contain only ingredients with GRAS (Generally Recognized as Safe) status by the FDA (as supported by robust dossiers of consensus scientific support, including published direct human outcome data and toxicology data), and are labeled for the dietary management of a specific disease or condition that has distinctive nutritional requirements. These products are expressly required to follow “good scientific principles,” which broadly includes being supported by well controlled clinical and scientific studies (using the formulation in the finished product) recognized by experts in the field, such as peer review in recognized medical and scientific journals. This raises the bar for natural products well beyond what is currently required to produce and market a product in the nutritional supplement regulatory category, where the manufacturer is extremely restricted in making any disease or health-related claims for the product, even when support for the individual ingredients may exist in the literature.
Conclusion
While there are many traditionally used natural agents with anti-inflammatory properties in naturopathic and integrative medicine, there are not many that are backed by rigorous scientific studies proving safety and efficacy and which meet the standards of classification as a medical food. Flavocoxid is one of these well-studied natural compounds, and provides the integrative clinician with a reliable, proven, low-side-effect profile. Flavocoxid is a natural fit for the dietary management of metabolic inflammatory processes in conditions such as osteoarthritis.

David M. Brady, ND has 22 years of experience as an integrative clinical practitioner and over 18 years in health sciences academia as faculty and an administrator. He currently serves as the Vice Provost for the Division of Health Sciences, Director of the Human Nutrition Institute, and Associate Professor of Clinical Sciences at the University of Bridgeport in Connecticut. Dr Brady is the Chief Medical Officer for Designs for Health, Inc., is an expert consultant to the nutritional supplement and clinical laboratory industries, and is an internationally sought-after presenter on nutritional, naturopathic and integrative medicine. He has appeared on the plenary speaking panel of some of the largest and most prestigious conferences in the field, such as, IFM, ACAM, A4M, IHS, AANP, NWANP, and ACA-CON. Dr Brady has published numerous scientific papers and textbooks related to chronic pain, autoimmunity and functional gastroenterology and is a featured contributing author in the medical textbooks: Advancing Medicine with Food and Nutrients, 2nd Ed. (Kohlstadt I. Boca Raton, FL: CRC Press; 2012), Integrative Gastroenterology (Mullin G. New York, NY: Oxford Press, Weil Integrative Medicine Library; 2011) and Laboratory Evaluations for Integrative and Functional Medicine, 2nd Ed. (Bralley JA, Lord RS.. Duluth, GA: Metametrix Institute; 2008).
References
- Singh G, Ramey DR, Morfeld D, et al. Gastrointestinal tract complications of non-steroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med. 1996;156(14):1530-1536.
- Nasrallah R, Robertson SJ, Karsh J, Hebert RL. Celecoxib modifies glomerular basement membrane and podocytes in OVE26 mice, but ibuprofen is more detrimental. Clin Sci (Lond). 2013; 124(11):685-694.
- Brady DM, Paul C. Anti-inflammatory options: Vioxx and other secretive COX-2 inhibitors v natural agents. Nutr Perspectives. 2009;32(2):33-36.
- Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of anti-inflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993;38(2-3):113-119.
- Safayhi H, Mack T, Sabieraj J, et al. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther. 1992;261(3):1143-1146.
- Ammon HP, Mack T, Singh GB, Safayhi H. Inhibition of leukotriene B4 formation in rat peritoneal neutrophils by an ethanolic extract of the gum resin exudate of Boswellia serrata. Planta Med. 1991;57(3):203-207.
- Safayhi H, Sailer ER, Ammon HP. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol Pharmacol. 1995;47(6):1212-1216.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13(1-4):225-230.
- Ammon HP. [Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases]. [Article in German] Wien Med Wochenschr. 2002;152(15-16):373-378.
- Wallace JM. Nutritional and botanical modulation of the inflammatory cascade–eicosanoids, cyclooxygenases, and lipoxygenases–as an adjunct in cancer therapy. Integr Cancer Ther. 2002;1(1):7-37; discussion 37.
- Kiuchi F, Iwakami S, Shibuya M, et al. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull (Tokyo). 1992;40(2):387-391.
- Koo KL, Ammit AJ, Tran VH, et al. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb Res. 2001;103(5):387-397.
- Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, et al. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res. 2003;111(4-5):259-265.
- Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40(8)1091-1097.
- Murakami A, Takahashi D, Kinoshita T, et al. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the alpha,beta-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23(5):795-802.
- Murakami A, Hayashi R, Tanaka T, et al. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combination. Biochem Pharmacol. 2003;66(7):1253-1261.
- Lumb AB. Effect of dried ginger on human platelet function. Thromb Haemost. 1994;71(1):110-111.
- Janssen PL, Meyboom S, van Staveren WA, et al. Consumption of ginger (Zingiber officinale roscoe) does not affect ex vivo platelet thromboxane production in humans. Eur J Clin Nutr. 1996;50(11):772-774.
- Verma SK, Singh J. Effect of ginger on platelet aggregation in man. Indian J Med Res. 1993;98:240-242.
- Srivastava KC. Effect of onion and ginger consumption on platelet thromboxane production in humans. Prostaglandins Leukot Essent Fatty Acids. 1989;35(3):183-185.
- Bierhaus A, Zhang Y, Quehenberger P, et al. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77(4):772-782.
- Shah BH, Nawaz Z, Pertani SA, et al. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol. 1999;58(7):1167-1172.
- Srivastava KC, Bordia A, Verma SK. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot Essent Fatty Acids. 1995;52(4):223-227.
- Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993;38(2-3):113-119.
- Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172(2):111-118.
- Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol. 2002;40(8)1091-1097.
- Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480-481:243-268.
- Sharma RA, Ireson CR, Verschoyle RD, et al. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: relationship with drug levels. Clin Cancer Res. 2001;7(5):1452-1458.
- Rao CV, Kawamori T, Hamid R, Reddy BS. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis. 1999;20(4):641-644.
- Pendurthi UR, Williams JT, Rao LV. Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr-1, AP-1, and NF-kappa B. Arterioscler Thromb Vasc Biol. 1997;17(12):3406-3413.
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24(12):651-654.
- Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. Med Assoc Thai. 1989;72(11):613-620.
- Kobayashi T, Hashimoto S, Horie T. Curcumin inhibition of Dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem Pharmacol. 1997;54(7):819-824.
- Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003;26(7):1021-1024.
- Masuda T, Hidaka K, Shinohara A, et al. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J Agric Food Chem. 1999;47(1):71-77.
- Kapoor S, Priyadarsini KI. Protection of radiation-induced protein damage by curcumin.
- Biophys Chem. 2001;92(1-2):119-126.
- Sun YM, Zhang HY, Chen DZ, Liu CB. Theoretical elucidation on the antioxidant mechanism of curcumin: a DFT study. Org Lett. 2002;4(17):2909-2911.
- Masuda T, Toi Y, Bando H, et al. Structural identification of new curcumin dimers and their contribution to the antioxidant mechanism of curcumin. J Agric Food Chem. 2002;50(9):2524-2530.
- Luo F, Huang R, Yang YJ, Yu XH. [Protective effect and mechanism of pretreatment with curcumin on infectious brain edema in rats.] [Article in Chinese] Zhonghua Er Ke Za Zhi. 2003;41(12):940-944.
- Aleksandrov PN, Speranskaia TV, Bobkov IuG, et al. [Effect of rutin and esculamine on models of aseptic inflammation.] [Article in Russian] Farmakol Toksikol. 1986;49(1):84-86.
- Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56(9):683-687.
- Olszanecki R, Gebska A, Kozlovski VI, Gryglewski RJ. Flavonoids and nitric oxide synthase. J Physiol Pharmacol. 2002;53(4 Pt 1):571-584.
- Kiho T, Usui S, Hirano K, et al. Tomato paste fraction inhibiting the formation of advanced glycation end-products. Biosci Biotechnol Biochem. 2004;68(1):200-205.
- Vadas P, Stefanski E, Pruzanski W. Potential therapeutic efficacy of inhibitors of human phospholipase A2 in septic shock. Agents Actions. 1986;19(3-4):194-202.
- Gryglewski RJ, Korbut R, Robak J, Swies J. On the mechanism of antithrombotic action of flavonoids. Biochem Pharmacol. 1987;36(3):317-322.
- Swies J, Robak J, Dabrowski L, et al. Antiaggregatory effects of flavonoids in vivo and their influence on lipoxygenase and cyclooxygenase in vitro. Pol J Pharmacol Pharm. 1984;36(5):455-463.
- Aleksandrov PN, Speranskaia TV, Bobkov IuG, et al. [Effect of rutin and esculamine on models of aseptic inflammation]. [Article in Russian] Farmakol Toksikol. 1986;49(1):84-86.
- Guardia T, Rotelli AE, Juarez AO, Pelzer AE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56(9):683-687.
- Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33(12):1061-1080.
- Stefanescu M, Matache C, Onu A, Szegli G. Modulation of cell adhesion by tyrosine kinases and phosphatases inhibitors. Roum Arch Microbiol Immunol. 1997;56(1-2):3-15.
- Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265(2 Pt 2):H774-778.
- Otsuka H, Inaba M, Fujikura T, Kunitomo M. Histochemical and functional characteristics of metachromatic cells in the nasal epithelium in allergic rhinitis: studies of nasal scrapings and their dispersed cells. J Allergy Clin Immunol. 1995;96(4):528-536.
- Pearce FL, Befus AD, Bienenstock J. Mucosal mast cells. III. Effect of quercetin and other flavonoids on antigen-induced histamine secretion from rat intestinal mast cells. J Allergy Clin Immunol. 1984;73(6):819-823.
- Middleton E Jr, Drzewiecki G. Flavonoid inhibition of human basophil histamine release stimulated by various agents. Biochem Pharmacol. 1984;33(21):3333-3338.
- Kimata M, Shichijo M, Miura T, et al. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000;30(4):501-508.
- O’Prey J, Brown J, Fleming J, Harrison PR. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem Pharmacol. 2003;66(11):2075-2088.
- Thuillier P, Brash AR, Kehrer JP, et al. Inhibition of peroxisome proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. Biochem J. 2002;366(Pt 3):901-910.
- Da Silva EL, Abdalla DS, Terao J. Inhibitory effect of flavonoids on low-density lipoprotein peroxidation catalyzed by mammalian 15-lipoxygenase. Life. 2000;49:289-295.
- Miodini P, Fioravanti L, Di Fronzo G, Cappellitti V. The two phyto-oestrogens genistein and quercetin exert different effects on oestrogen receptor function. Br J Cancer. 1999;80(8):1150-1155.
- Koga T, Meydani M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am J Clin Nutr. 2001;73(5):941-948.
- de Whalley CV, Rankin SM, Hoult JR, et al. Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biochem Pharmacol. 1990;39(11):1743-1750.
- Teixeira S. Bioflavonoids: proanthocyanidins and quercetin and their potential roles in treating musculoskeletal conditions. J Orthop Sports Phys Ther. 2002;32(7):357-363.
- Lo AH, Liang YC, Lin-Shiau SY, et al. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23(6):983-991.
- Chan MM, Ho CT, Huang HI. Effects of three dietary phytochemicals from tea, rosemary and turmeric on inflammation-induced nitrite production. Cancer Lett. 1995;96(1):23-29.
- Huang MT, Ho CT, Wang ZY, et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54(3):701-708.
- Wargovich MJ, Woods C, Hollis DM, Zander ME. Herbals, cancer prevention and health. J Nutr. 2001;131(11 Suppl):3034S-3036S.
- Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104(1):43-48.
- Kosaka K, Yokoi T.Carnosic acid, a component of rosemary (Rosmarinus officinalis L.), promotes synthesis of nerve growth factor in T98G human glioblastoma cells. Biol Pharm Bull. 2003;26(11):1620-1622.
- Danilenko M, Wang X, Studzinski GP. Carnosic acid and promotion of monocytic differentiation of HL60-G cells initiated by other agents. J Natl Cancer Inst. 2001;93(16):1224-1233.
- Valenzuela A, Sanhueza J, Nieto S. Cholesterol oxidation: health hazard and the role of antioxidants in prevention. Biol Res. 2003;36(3-4):291-302.
- Debersac P, Vernevaut MF, Amiot MJ. Effects of a water-soluble extract of rosemary and its purified component rosmarinic acid on xenobiotic-metabolizing enzymes in rat liver. Food Chem Toxicol. 2001;39(2):109-117.
- Singletary KW, Rokusek JT. Tissue-specific enhancement of xenobiotic detoxification enzymes in mice by dietary rosemary extract. Plant Foods Hum Nutr. 1997;50(1):47-53.
- Singletary KW. Rosemary extract and carnosol stimulate rat liver glutathione-S-transferase and quinone reductase activities. Cancer Lett. 1996;100(1-2):139-44.
- Del Campo J, Amiot MJ, Nguyen-The C. Antimicrobial effect of rosemary extracts. J Food Prot. 2000;63(10)1359-1368.
- Culpitt SV, Rogers DF, Fenwick PS, et al. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58(11):942-946.
- Cavallaro A, Ainis T, Bottari C, Fimiani V. Effect of resveratrol on some activities of isolated and in whole blood human neutrophils. Physiol Res. 2003;52(5):555-562.
- Ignatowicz E, Baer-Dubowska W. Resveratrol, a natural chemopreventive agent against degenerative diseases. Pol J Pharmacol. 2001;53(6):557-569.
- Bertelli AA, Baccalini R, Battaglia E, et al. Resveratrol inhibits TNF alpha-induced endothelial cell activation. Therapie. 2001;56(5):613-616.
- Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164(12):6509-6519.
- Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126(3):673-680.
- Pace-Asciak CR, Hahn S, Diamandis EP, et al. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235(2):207-219.
- Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem Pharmacol. 2003;65(5):773-781.
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2.
- N Engl J Med. 2001;345(6):433-442.
- De Caterina R, Zampolli A. From asthma to atherosclerosis–5-lipoxygenase, leukotrienes, and inflammation. N Engl J Med. 2004;350(1):4-7.
- USDA National Nutrient Database for Standard Reference. Updated December 11, 2011. http://ndb.nal.usda.gov/. Accessed May 13, 2013.
- Middleton E Jr., Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673-751.
- Jia Q. Generating and screening a natural product library for cyclooxygenase & lipoxygenase dual inhibitors. Studies in Natural Product Chemistry. 2003;29(Part J):643-718.
- Burnett BP, Jia Q, Zhao Y, nd Levy RM. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J Med Food. 2007;10(3):442-451.
- Burnett BP, Bitto A, Altavilla D, et al. Flavocoxid inhibits phospholipase A2, peroxidase moieties of the cyclooxygenases (COX), 5-lipoxygenase, modifies COX-2 gene expression and acts as an antioxidant. Mediators Inflamm. 2011;2011:385780.
- Sampalis JS, Brownell LA. A randomized, double blind, placebo and active comparator controlled pilot study of UP446, a novel dual pathway inhibitor anti-inflammatory agent of botanical origin. Nutr J. 2012;11:21.
- Pillai L, Burnett BP, Levy RM, et al. GOAL: multicenter, open-label, post-marketing study of flavocoxid, a novel dual pathway inhibitor anti-inflammatory agent of botanical origin. Curr Med Res Opin. 2010;26(5):1055-1063.
- Chalasani N, Vuppalanchi R, Navarro V, et al. Acute liver injury due to flavocoxid (Limbrel), a medical food for osteoarthritis: a case series. Ann Intern Med. 2012;156(12):857-860.
- Panduranga V, Atienza J, Kumar A, Meteresky ML. Hypersensitivity pneumonitis due to flavocoxid: are corticosteroids necessary? Conn Med. 2013;77(2):87-90.
- Morgan SL, Baggott JE, Moreland L, et al. The safety of flavocoxid, a medical food, in the dietary management of knee osteoarthritis. J Med Food. 2009;12(5):1143-1148.
- Levy RM, Khokhlov A, Kopenkin S, et al. Efficacy and safety of flavocoxid, a novel therapeutic, compared with naproxen: a randomized multicenter controlled trial in subjects with osteoarthritis of the knee. Adv Ther. 2010;27(10):731-742.
- Altavilla D, Squadrito F, Bitto A, et al. Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin stimulated macrophages. Brit J Pharmacol. 2009;157(8):1410-1418.
- Messina S, Bitto A, Aquennouz M, et al. Flavocoxid counteracts muscle necrosis and improves functional properties in mdx mice: a comparison study with methylprednisolone. Exp Neurol. 2009;220(2):349-358.
- Levy RM, Saikovsky R, Shmidt E, et al. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: a short-term randomized, double-blind pilot study. Nutr Res. 2009;29(5):298-304.
- Pillai L, Levy RM, Yiman M, et al. Flavocoxid, an anti-inflammatory agent of botanical origin, does not affect coagulation or interact with anticoagulation therapies. Adv Ther. 2010;27(6):400-411.
- Polito F, Irrera N, Bitto A, et al. 2011. Flavocoxid, a dual inhibitor of COX and 5-LOX, attenuates inflammation and bone resorption in a mouse model of collagen induced arthritis. Submitted. (Data on file at Primus Pharmaceuticals, Inc., Scottsdale, AZ.)
- Polito F, Bitto A, Irrera N, et al. Flavocoxid, a dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, reduces pancreatic damage in an experimental model of acute pancreatitis. Br J Pharmacol. 2010;161(5):1002-1011.
- Suggs-Anderson S. Regulation of medical Foods [presentation]. 2012 FDA Rare Disease Patient Advocacy Day. March 1, 2012. U.S. FDA Web site. http://www.fda.gov/downloads/ForIndustry/DevelopingProductsforRareDiseasesConditions/OOPDNewsArchive/UCM292733.pdf. Accessed May 14, 2013.