Cora Stover, ND
Diana Zitserman, ND
Radley M. Ramdhan
Naturopathic Perspective
Traumatic Brain Injury, or TBI, has been noted in history, literature, and medical societies for quite some time. Boericke noted it in one of his “Never well since…” rubrics, along with other rubrics. In 1924, Cassasa was recognized as the first to describe the phenomenon and what repeated traumatic injuries might do to a person, and coined the term dementia pugillistica (or DP). Cassasa observed that boxers who were being used for training purposes would eventually display a particular pattern of symptoms.1
These symptoms first appeared in the extremities, as a slight flopping of the foot or leg, only noticeable upon walking or at intervals, or as a slight unsteadiness in gait or equilibrium. A peculiarity was the fact that these fighters could fight exceptionally well, but as soon as they stopped or walked back to their corner, the abnormality became apparent. Further symptomology included confusion and muscular slowing. Other terms historically associated with this phenomenon, which were generally applied to an intoxicated person, included: Punch Drunk, Cuck-coo, Goofy, cutting paper dolls, and slug nutty.1 Cartoons often served to depict the syndrome; some famous sayings and characters were based on this association.
Chronic Traumatic Encephalopathy
Dr Bennet Omalu is world-renowned physician and neuroscientist who has been investigating the effects of repetitive brain injuries in professional sports players. It was a Professor Henry Miller who in 1966 coined the term Chronic Traumatic Encephalopathy (CTE) for the progressive neurodegenerative syndrome due to repetitive concussions and/or traumatic brain injuries.2 Omalu was the first to publish evidence of the syndrome in 2005.3 CTE is 1.5 times more likely to be seen in males than females.4 First discovered in the brain of NFL Hall-of-Famer, Mike Webster, in 2002, CTE was subsequently identified in NFL players, wrestlers, hockey players, deceased military veterans, domestic abuse victims, motor vehicle accident sufferers, and others.5 Bennet Omalu’s story was portrayed in the 2015 cinematic hit, Concussion, and his growing foundation and published work has continued to spread throughout the scientific and medical community.
CTE is a distinctive disease, and often a sequela to TBI. Clinically, CTE presents as a combined syndrome featuring mood disorders, behavioral and/or cognitive impairment, and occasional sensorimotor involvement. CTE is caused by “single, episodic, or repetitive blunt force trauma to the head, with repeated acceleration-deceleration forces to the brain.”5 A CTE patient’s brain appears largely unremarkable; however, microscopic examination of post-mortem brains reveals primary and secondary proteinopathies. Dr. Omalu found that the primary proteinopathy of CTE is tau protein-related, whereas secondary proteinopathies include amyloid plaque and other abnormalities. Dr Omalu and his team have pioneered the study of CTE-related proteinopathies by developing an in-vivo PET scan named FDDNP-PET, which measures tau tangles and amyloid plaque deposition in living tissue.5
Gross Morphology & Pathophysiology
Areas within the brain that are thought to be damaged in CTE include: the corpora striata (part of the basal ganglia comprising the caudate nucleus [which contains endorphins] and the lentiform nucleus, which when probed produce sensations of thirst; its function is essentially motor); the corona radiata (a sheet of white matter that runs ventrally as the internal capsule and dorsally as the semioval center – the corona radiata is responsible for most of the neural traffic to and from the cerebral cortex); the cerebral cortex (gray matter, which plays an important role in consciousness, thought and action; it includes 4 lobes: frontal, parietal, occipital, and temporal) or below the tentorium cerebelli (extension of the dura mater that separates the cerebellum from the occipital lobes).6
The pathology of DP and CTE are thought to involve hemorrhages that are later replaced by gliosis, a degenerative progressive lesion. In 1924, Cassasa described these areas like this:
At autopsy, sections of the brain showed multiple usually punctate hemorrhages scattered over various parts of the parenchyma of the brain. Lacerations of the scalp, fractures of the skull, cortical lacerations or hemorrhages – except for occasional pia-arachnoid hemorrhages – were not found. Microscopic examination showed these punctate hemorrhages to be located around the blood vessels in the perivascular spaces of Virchow-Robin [an immunological space around an artery and vein – not capillary, and pia mater that may be expanded by leukocytes].1
Clinical Manifestation, Diagnosis & Testing
Diagnosing brain injury can be extensive and expensive. Two scales which should be used in any case of brain injury include the Glasgow Coma Scale and the Ranchos Los Amigos Scale. Imaging, including angiography, computed tomography (CT), magnetic resonance imaging (MRI), and X-rays, should be utilized to observe patterns of brain injury due to external and internal forces or other possible traumas. Functional imaging, such as electroencephalography (EEG) and transcranial doppler, may be utilized to observe communication and neural patterns. Because biofeedback can pick up changes in autonomic nervous system function that can occur during inflammation and encephalopathy, it might assist in our understanding and treatment of TBI/CTE.7,8
Clinical manifestation of TBI sequelae may include the signs and symptoms shown in Table 1.
Table 1. Possible Clinical Sequelae of TBI6,7,8
| Unconsciousness (left side injury lasting longer vs right side) | Behavioral/mood changes (display of pathological jealousy/ paranoia) | Difficulty with memory, concentration, attention, thinking |
| Dilation of one or both pupils (anisocoria) | Difficulty with processing speed and executive functioning | Cushing’s Triad: Depressed HR, irregular respirations, and increased BP |
| Alexithymia (inability to identify, understand, process, and describe emotions) | Lack of motor coordination/balance | Nausea/vomiting |
| Blurred vision/double vision | Ringing in ears | Confusion |
| Bad taste in mouth | Fatigue/lethargy | Headache |
| Convulsions | Dizziness | Sleep pattern changes |
| Slurred speech | Aphasia, dysarthria | Weakness/numbness in limbs |
| Social judgment deficits | Concussion, post-concussion syndrome |
In addition, signs and symptoms in young children may include persistent crying, inability to be consoled, listlessness, refusal to nurse/eat, irritability, and behavior changes.9
Suggested biomarkers include a CSF:serum albumin ratio (which can reveal blood-brain barrier dysfunction); neuro-inflammatory cytokines such as IL-6, IL-8 and IL-10 (typically increased after severe TBI); and other acute-phase response proteins.10 Acute axonal injury biomarkers include total tau and neurofilament light polypeptide (NFL), which tend to increase 4-10 days after injury; these 2 tests are considered most accurate and to correlate with injury. Myelin basic protein (measured in blood) is also associated with TBI. Another promising marker for acute neuronal damage is CSF measurement of γ-Enolase, also known as neuron-specific enolase (NSE); this is a glycolytic enzyme in neuronal cell bodies and is also present in erythrocytes and endocrine cells. Calpain and caspase (breakdown products of neuronal spectrin a chain [αII spectrin]) tend to increase in the CSF during acute-phase brain injuries.10 Serum S-100β may be a useful marker of astrocyte injury or death and correlates with the Glasgow Coma Scale; however, it is not useful in children under 2 years of age.11
Pathogenesis of Neuroinflammation
In order to understand CTE, it is helpful to first explore the pathogenesis of TBI-related neuroinflammation. TBI is generalized into 4 categories, as follows: “1) primary injury that disrupts brain tissues; 2) secondary injury that causes pathophysiology in the brain; 3) inflammatory response that adds to neurodegeneration; and 4) repair-regeneration [of neurons].”12 A primary injury to the brain initiates the secondary injury, or the cascade of multiple molecular events that lead to neurodegeneration.
In the first hours after TBI, in cases of focal injury, damaged cells are found in clusters. In cases of diffuse injury, these changes are more diffuse in the brain. Sites of injury contain both necrotized neurons and non-neuronal cells, and hemorrhagic areas can become intraparenchymal hematomas in cases of more extensive injury.13 High ventricular intracranial pressure after TBI can indicated a poor outcome.12 Varying degrees of edema and cellular swelling can also be present, and a broad, asymmetrical distribution of axon swelling can indicate diffuse axonal damage.13 TBI and cellular responses are outlined in Figure 1.
Figure 1. TBI & Cellular Effects on Brain Tissue13
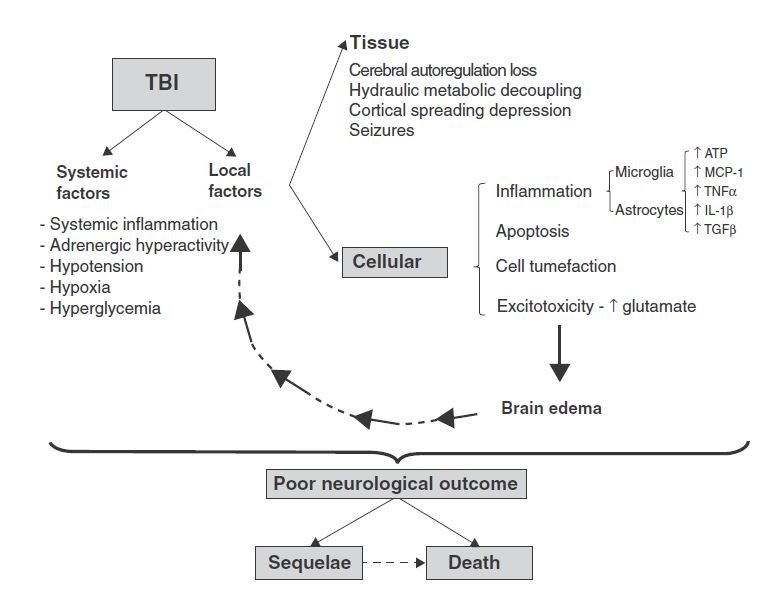
Secondary mediators are known to be tissue-destructive, involving inflammation and apoptosis.14 TBI is often associated with a breached blood-brain barrier (BBB), which can be accompanied by endothelial activation and recruitment of white blood cells, as well as microglial activation and migration toward the injury. This can result from a number of mechanisms, such as chemoattractant protein-1 (MCP-1) and cytokines, eg, transforming growth factor-beta (TGFβ). Release of tumor necrosis factor-alpha (TNFα) and interleukin (IL)-1-beta (IL-1-β) in turn promote increased expression of an intermediate filament called glial fibrillary acidic protein (GFAP). Leukocytes and activated cells release proinflammatory cytokines, nitric oxide, and free oxygen radicals, which introduces phenotypic changes called “neuroplastic remodeling,” in which increased branching of dendrites occur to form new synaptic connections.13
Other common features include intracellular increases in free calcium, disturbances in ion concentration (eg, increased potassium or decreased magnesium), acetylcholine deficiency (particularly in chronic cases), dynorphin (an endogenous opioid receptor correlated with regional neurodegeneration), nitric oxide (induced by ischemic damage), and calcium-dependent cysteine proteases including calpain and caspase-3 (which are contributors to cell death following TBI).12,15 In more severe TBI, astrocytosis can lead to tissue scarring, impairing structure and function of the brain areas involved in the trauma.13
Another key player is the excitotoxicity and spread of apoptosis endured by the traumatized tissues. NMDA receptor activation is responsible for excitatory signals. In fact, it has been documented that TBI-induced glutamate excitotoxicity is the initiating signal for apoptosis of damaged cell membranes. Increased extracellular glutamate levels activate glutamate receptors, leading to intracellular calcium overload, which stimulates mitochondrial oxidative stress and activation of the caspase-dependent and independent apoptosis, ie, delayed cell death.15 Other studies also suggest that a heterodimer formation of pro-apoptotic transcription factors, such as c-Fos and c-Jun, can regulate the expression of genes coding for opioid peptides, amyloid beta protein, and nerve growth factor, which have been shown to be overexpressed in TBI.12
TBI and CTE appear to represent a progressive neurodegenerative state in which tau and amyloid beta proteins accumulate within the tissues over time. These molecular mechanisms have been shown to be initiated by the extrasynaptic NMDA activation that produces soluble amyloid-beta, and caspase-3 activation that leads to formation of tau neurofibrillary tangles.15 As we know, amyloid proteinopathy is associated with Alzheimer’s disease (AD), and tau-related neurofibrillary tangles are associated with Parkinson’s disease (PD). Therefore, CTE post-TBI can be a concomitant condition with dementia, AD, and PD.
Treatment of TBI
Treatment of TBI varies, based on its severity. These range from simple measures such as rest in mild TBI cases, to surgery in more severe cases. Additional acute and chronic treatments exist, as well as long-term rehabilitation options. Initial acute treatments for TBI may include clearing the lungs to aid breathing, maintaining healthy circulation to support and protect the brain and other vital organs, monitoring heart rate, medicating the patient to reduce risk of secondary damage, and removing any blood clots that may result in patient’s life being compromised. Chronic treatment and long-term rehabilitation measures can include assistive technology, counseling and/or therapy, medication, physical therapy, or speech therapy.7
Allopathic Approach
Management of a sports-related concussion often includes cognitive rest, physical rest, medication, and a gradual transition back into playing.7 Cognitive rest involves avoidance of activities that require extra attention such as text messaging, video games, television, computer use, or school work. Physical rest is encouraged to avoid exacerbation of symptoms. NSAIDs, acetaminophen, or amitriptyline may be used for persistent headaches; sleep medications, anxiolytics, and SSRIs may be utilized for depressive symptoms. Transition back to play is also an important part of the process where it is a step-by-step process from non-impact to full contact over a period of days, with the patient being in a symptom-free state.7
Naturopathic Approach
Our focus as naturopathic doctors should be on treating with natural anti-inflammatories, adaptogens, neuroprotective and neuro-regenerative agents, and analgesics. This can be accomplished through herbs, diet, nutraceuticals, homeopathy, and other modalities. Treatments can also be approached with different strategies in mind. These may include reducing the short- and long-term impacts of inflammation on brain tissues; supporting regrowth of brain tissues and optimal neuronal function; managing peripheral symptoms such as tremor, palsy, or neuropathy; optimizing circulation to the brain to reduce inflammation and promote regeneration (especially in cases involving hemorrhage), and managing any associated neuropsychiatric symptoms.
The short- and long-term impacts of inflammation on the brain can be reduced by plant flavonoids such as anthocyanidins from blueberry (Vaccinium macrocarpon), glycosylated flavonoids such as ginkgo (Ginkgo biloba) flavone glycosides, curcumin (Curcuma longa), boswellia (Boswellia serata), and omega-3 essential fatty acids. Oats (Avena sativa), rich in calmodulin and phosphatidylinositol, can be used to improve nerve function and promote regrowth of the brain tissues. Mullein (Verbascum thapsus) contains piscicide, which has analgesic effects. Valerian (Valeriana officinalis) has anticonvulsant effects, and Huperzia serrata has nootropic and neuroprotective effects and has been seen to potentiate the effects of acetylcholine. These are all herbs that can be used internally to help manage any peripheral symptoms.16
External application of St John’s wort (Hypericum perforatum) or chili pepper (Piper frutescens) have both demonstrated effectiveness in treating neuropathy. Herbs such as hawthorn (Crataegus spp), rosemary (Rosmarinus officinalis), linden (Tilia spp) and ginger (Zingiber officinale) can be utilized to optimize circulation, helping to reduce inflammation and promote regeneration. Rhodiola (R rosea) and ashwagandha (Withania somnifera) may be utilized for their adaptogenic properties as well as their ability to support the brain in the case of associated neuropsychiatric symptoms. St John’s wort can also be used for its antidepressant effects. Skullcap (Scutellaria lateriflora), lemon balm (Melissa officinalis), kava kava (Piper methysticum), and Bacopa monnieri are all herbs that can be utilized as nervines and to manage any associated neuropsychiatric symptoms.16
Homeopathic remedies that can be useful for TBI include Arnica, Belladonna, Hypericum, Cicuta, Natrum sulphuricum, and Helleborus. Arnica is useful in those who deny any problems following the trauma. Belladonna is useful when there is significant heat, redness, throbbing, and fullness in the head after an injury. Hypericum can be effective in patients with sharp and shooting pains, spasms, or seizures after a head injury. Cicuta is useful in cases where seizures occur following the head injury, or the head injury is severe enough to have caused mental retardation. Nat Sulph is useful for long-term symptoms following a head injury that includes personality changes. Helleborus is useful in a patient that develops severe mental dullness and may talk very slowly after the head injury.17
Hyperbaric Oxygen Therapy (HBOT) is also a useful treatment for TBI. HBOT involves the medical therapeutic use of oxygen at a high atmospheric pressure. It can be used for both acute and chronic TBI patients; however, some studies have revealed that it has been more effective in the chronic TBI patient groups.18,19
Historically, the Roman Army utilized herbs as part of their treatment plan for soldiers with TBI. Tea made from Glaucium corniculatum was sometimes used because it acts as an acetylcholinesterase inhibitor.20 A decoction of the roots of Asparagus officinalis was used to reduce edema. A mixture of acetum (vinegar) and the juice of the plant Glaucium flavum was used to clean the wound area; this was followed by a dressing containing a mixture of honey, lint, and Aloe vera. Diet and hydrotherapy also played roles in the treatment plans. In more severe cases of TBI, Papaver somniferum or Mandragora officinarium was used for analgesia. In patients with weaker constitutions, Hyoscyamus niger combined with Papaver somniferum was sometimes used as an anesthetic.20
In addition to support with herbs, nutrition, homeopathy and hyperbaric oxygen therapy, it is important to support the natural healing process by ensuring that patients get ample rest and have a gradual phase of return to the sports that they were playing prior to their TBI. And, of course, it is vital to initially take a proper case history and perform a comprehensive physical exam in order to gather the information that will help determine the best treatment plan.
References:
- Martland HS. Punch Drunk. JAMA. 1928;91(15):1103-1107.
- Miller H. Mental after-effects of head injury. Proc R Soc Med. 1966;59(3):257-261.
- Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005 Jul;57(1):128-34; discussion 128-34.
- Faul M, Xu L, Waid MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. March 2010. CDC Web site. https://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. Accessed April 4, 2016.
- Omalu B. Chronic traumatic encephalopathy. Prog Neurol Surg. 2014;28:38-49.
- McCaffrey P. Chapter 11. Traumatic Brain Injury: Effects of Closed Head Injury. 1998-2008. The Neuroscience on the Web Series: CMSD 636 Neuropathologies of Language and Cognition. CSU Chico. Available at: https://www.csuchico.edu/~pmccaffrey/syllabi/SPPA336/336unit11.html. Accessed April 4, 2016.
- Scorza KA, Raleigh MF, O’Connor FG. Current concepts in concussion: evaluation and management. Am Fam Physician. 2012;85(2):123-132.
- Brain Injury in Children. Brain Injury Association of America. 2015. Available at:http://www.biausa.org/brain-injury-children.htm#symptoms. Accessed April 4, 2016.
- Nordqvist C. Traumatic Brain Injury: Causes, Symptoms and Diagnosis. Last updated March 4, 2016. MedicalNewsToday Web site. https://www.medicalnewstoday.com/articles/179837.php. Accessed April 4, 2016.
- Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9(4):201-210.
- Mondello S, Muller U, Jeromin A, et al. Blood‐based diagnostics of traumatic brain injuries. Expert Rev Mol Diagn. 2011;11(1):65-78.
- Ray SK, Dixon CE, Banik NL. Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol. 2002;17(4):1137-1152.
- Rovegno M, Soto PA, Sáez JC, von Bernhardi R. Biological mechanisms involved in the spread of traumatic brain damage. Med Intensiva. 2012;36(1):37-44. [Article in Spanish]
- Veenith T, Goon SSh, Burnstein RM. Molecular mechanisms of traumatic brain injury: the missing link in management. World J Emerg Surg. 2009;4:7.
- Walker KR, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front Aging Neurosci. 2013;5:29.
- Mase G. Herbal Support for Traumatic Brain Injury: General recommendations and specific strategies. 2011. Available at: http://www.vtherbcenter.org/wp-content/uploads/2012/04/Herbs-for-TBI.pdf. Accessed April 4, 2016.
- Ullman D. Homeopathic Medicines for Traumatic Brain Injury. April 15, 2009. Natural News Web site. <http://www.naturalnews.com/026057_injury_homeopathic_medicines.html#. Accessed April 4, 2016.
- Stoller KP. Hyperbaric oxygen therapy (1.5 ATA) in treating sports related TBI/CTE: two case reports. Med Gas Res. 2011;1(1):17.
- Lin JW, Tsai JT, Lee LM, et al. Effect of hyperbaric oxygen on patients with traumatic brain injury. Acta Neurochir Suppl. 2008;101:145-149.
- Belfiglio VJ. Treatment of Traumatic Brain Injury in the Roman Army. Balkan Military Medical Review. 2015;18(4):101-105. Available at: https://www.ejmanager.com/mnstemps/42/42-1431537627.pdf. Accessed April 4, 2016.
Image Copyright: <a href=’https://www.123rf.com/profile_olegdudko’>olegdudko / 123RF Stock Photo</a>
 Cora Stover, ND, is a doctor of Naturopathic Medicine who provides services to the New Haven and Fairfield counties and is bringing medicine back into the home via home-health. She enjoys working with a variety of patients and conditions. Her passions include laughing, gardening, learning, and being mystified by nature and our experiences.
Cora Stover, ND, is a doctor of Naturopathic Medicine who provides services to the New Haven and Fairfield counties and is bringing medicine back into the home via home-health. She enjoys working with a variety of patients and conditions. Her passions include laughing, gardening, learning, and being mystified by nature and our experiences.
***
Diana Zitserman, ND, is a licensed naturopathic physician and acupuncturist, specializing in Whole Person Wellness at Collaborative Natural Health Partners in Manchester, CT. After completing her studies at Pennsylvania State University, Dr Zitserman pursued a 7-year research career at Fox Chase Cancer Center, which included 8 journal publications. Furthering her medical studies in naturopathy and Traditional Chinese Medicine at the University of Bridgeport, she combined her love of science with an innate passion for understanding human interaction, nature, and alternative healing. Dr Z uses natural therapies to create balance and health restoration for people of all ages.
***
 Radley M. Ramdhan is currently working toward a Doctorate of Naturopathic Medicine and a Master of Science in Acupuncture at the University of Bridgeport; he anticipates graduation in May 2018. Serving in the New York Army National Guard since 2012, Radley recently returned from a deployment to Kuwait/Iraq for Operation Inherent Resolve 2016/2017. Radley has a passion for and desire to work with veteran and pediatric populations. Writing, traveling, and playing sports are some of Radley’s pleasures.
Radley M. Ramdhan is currently working toward a Doctorate of Naturopathic Medicine and a Master of Science in Acupuncture at the University of Bridgeport; he anticipates graduation in May 2018. Serving in the New York Army National Guard since 2012, Radley recently returned from a deployment to Kuwait/Iraq for Operation Inherent Resolve 2016/2017. Radley has a passion for and desire to work with veteran and pediatric populations. Writing, traveling, and playing sports are some of Radley’s pleasures.