Millie Lytle, ND, MPH, CNS
I have a plan to live healthy until 120 years old. And I am inviting my patients to join me. I believe this is a real possibility due to several factors entering social and scientific awareness. There is an increasing attention on centenarians. Each year approximately 17 in 100 000 Americans, mostly women, turn 100 years old. It has been reported that approximately 1 in 1000 centenarians will live past 110 years old, making them supercentenarians. Centenarians and supercentenarians are being studied as to why they have outlived their peers. A growing field of health research called epigenetics can allow us to morph susceptibilities traditionally thought impossible by altering the genome environment and tailoring the body’s natural defenses away from aging and towards youth. The field of epigenetics involves the quality and behavior of gene expression. Epigenetics is an emerging health field that finally provides a satisfactory response to the “nature versus nurture” debate. The answer is clear: “both.” Each human is a combination of the genetic material inherited from previous generations, and the result of contextual development including stress, nourishment, shelter, personality, mental outlook, and comorbid illness.As proposed by Dr Joseph Chang, PhD, in his book The Aging Myth, neither the side effects of aging (gray hair, wrinkles, poor virility, and low energy) nor age-related diseases are automatic outcomes of aging.1 Thus, aging itself may, in fact, be optional.
Longevity Syndrome
Longevity syndrome is an epigenetic-based condition defined as “alonger-than-expected life span seen in an autosomal dominant family cohort that lives ±10 years longer than average, due to a mutation in apolipoprotein A-1—ApoA-I Milano—which results in marked increased in HDL of greater than 75 mg/dL.”2For instance, though your patient may have a gene that predisposes him or her to heart disease via familial hypercholesterolemia, it does not mean that the disease is inevitable. Every other member of that high-risk family could have succumbed to the disease, with the exception of 1 individual. The genetic expression activity of individuals can be measured against their lifestyle to determine why they outlived the rest of their family. Dr Bruce Lipton, cell biologist and professor of medicine, writes in his book, The Biology of Belief, that an error in genetics research has been made in analyzing only the DNA itself without looking at the cytoplasm or the cellular material surrounding the genes.3 A gene may be present, but it will only be expressed under a certain set of circumstance or in a particular environment. The obvious relevance for naturopathic doctors is that environmental factors participating in the positive or negative expression of genes have been shown to include interventions such as nutrition, lifestyle, environmental exposure, and even quality of thought. Results from a Silician centenarian study showed that longevity is the result of an optimal performance of the immune system and an overriding of anti-inflammatory sequence variants of immune/inflammatory genes.4 Genetic, epigenetic, stochastic (random), and environmental factors seem to have crucial roles in aging and longevity, with epigenetics being associated with aging, as demonstrated in many studies.4 Meanwhile, evolutionary biologist, JJ Mitteldorf, has pooled biochemical and clinical research and proposed a theory of a new epigenetic circadian rhythm as the determinant for longevity in individuals and select communities.5 In opposition to the mainstream evolutionary viewpoint that the longevity potential of a human being is pre-programmed, he hypothesizes that the length of the telomere is the yardstick. Telomeres are the end-caps of chromosomes, whose shortening has been shown linked to conditions of aging, cancer, inflammation, and sequestration of telomerase in humans. Mitteldorf has proposed that a long telomere is the circadian clock of the human lifespan potential; the longer the telomere, the longer the life.5 Some basic dietary and lifestyle factors, including stress reduction, a whole-foods diet with low refined carbohydrates and less than 10% fat intake, as well as moderate exercise, have been shown to lengthen the telomeres of men with prostate cancer over a 5-year period in a recent pilot study led by Dr Dean Ornish, and published in the Lancet Oncology.6
With many positive incentives for further research, the most widely-understood epigenetic mechanism may be the methylation state of the genome as it relates to the body’s age; in particular, aging is associated with a global loss of methylation capacity.4 Methylation has been proposed as an appropriate target for anti-aging research,as it is backed up by specific experimental results that point in this direction.7
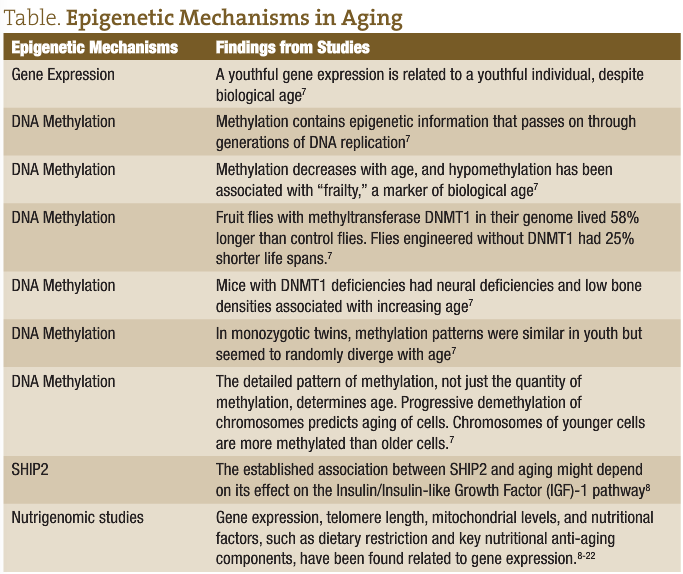
As further research has been done in the field of epigenetics, several laboratory, animal, and human studies have shown that a series of factors for genes called peroxisome proliferator-activated receptors (PPARs) are partially responsible for genetic “on” and “off” switches that control nutritionally-related chronic disease.9 PPARs act through the regulation of numerous biological processes, including fat and sugar metabolism and overall energy balance (homeostasis). Importantly, PPARs also mediate the inflammatory response, which makes them a possible therapeutic target for reducing obesity-induced inflammation and its serious health burden.10 PPAR activity has been implicated in both the cause and treatment of several chronic diseases, including atherosclerosis, diabetes, hyperglycemia, obesity, regulation of fertility, central nervous system conditions of myelination, various types of cancer, and more.11 In recent years, a lot of exciting research has shown nutrigenomic links to PPARs activation and gene expression to prevent and treat some of the most common chronic diseases.Nutrigenomics is an aspect of functional medicine and a science that studies the effects of food and nutrients on gene expression. For comparison purposes, general lifestyle factors such as exercise or coping with stress are included in epigenetic research, whereas Astragalus or a Chinese traditional formula called Shoushen Granules, both of which have been linked to lengthening telomeres, are studied within the field of nutrigenomics. Naturopathic doctors can apply the most current information on nutritional research to individualize treatment of patients living with or predisposed to particular genetic and nutritional conditions. Below is some research I’ve found to be the most interesting and poignant to the clinical practice of naturopathic medicine. The body of the research includes an accumulation of research on biochemical pathways such as DNA methylation and inflammation pathways, in vitro studies on the human genome, nutritional yeast, fruit flies, mice other small mammals, and clinical trials on humans. The research presented below is not a totality of the research but highlights some interesting and clinically useful examples of how far the research has come, as well as some possibilities for your practice. Naturopathic medicine has always relied on theoretical as well as clinical data to address patient conditions, and for naturopathic doctors it’s a perfect time to incorporate specific anti-aging science into your practice.
Dietary Restriction and Resveratrol
Dietary restriction (DR), which is marked by a limited calorie intake (as few as 1000-1200 calories per day), a low intake protein (10-18% of daily calories), and high-density nutrition from seeds, berries, and greens, has been shown to decrease IGF-1 levels, correlating to a younger cellular age and increased lifespan.12,13 The polyphenol resveratrol, known as the red wine antioxidant but sourced in higher quality and quantities from S cerevisiae and Japanese knotweed, may be a dietary mimetic of some effects of DR. Through specific effects on DNA methylation, the Sirtuin 1 (Sirt1) gene has been shown to have a pivotal role via the mammalian histone deacetylase in mediating the effects of both DR and resveratrol on lifespan/aging, suggesting that it may be the common conduit through which these dietary interventions influence aging.12,14 Researchers have proposed the novel hypothesis that effects of DR relevant to lifespan extension include maintenance of DNA methylation patterns through Sirt1-mediated epigenetic effects, and that dietary ingredients including resveratrol may mimic these actions. As this can be accomplished during adulthood as well as in utero, it appears that dietary components may influence and even predict longevity, from conception to adulthood.13
Pterostilbene
Pterostilbene is the brain antioxidant from the “brainberry,” blueberry (Vaccinium sp). Results from in vitro studies have shown that pterostilbene promotes PPAR-alpha and may be an effective lipid-reducer, even more effective than its cousin, resveratrol, for degenerative and sugar-related conditions of the brain. In vivo studies have demonstrated that this powerful antioxidant has cholesterol- and blood sugar-lowering effects.15 In another study, blood markers of cellular stress, inflammation, and Alzheimer’s disease pathology were positively lowered by pterostilbene via PPAR-alpha expression, which correlated with protection from age-related brain conditions. In summary, research findings indicate that doses of pterostilbene that are achievable in the diet and in supplement form are potent modulators of cognition, memory, and cellular stress, due to increased PPAR-alpha expression and fat-solubility of pterostilbene. Now that Alzheimer’s is emerging as “diabetes of the brain” or “type 3 diabetes,” this information is promising for the prevention of Alzheimer’s. especially when comorbid with sugar and insulin dysregulation that comes with metabolic syndrome, diabetes, and statin prescription.
Omega-3 and 6 Fatty Acids
Traditionally, chlorella has been recommended for its B vitamins, minerals, and detox factors; however, genetic studies are now also showing that chlorella contains fatty acids that are healthy for the genome. The bioactivities of several healthy fats contained within Chlorella sorokiniana were evaluated for their PPAR-gamma activity.17 Impressive results showed that linolenic acid (omega-3, algae DHA) and linoleic acid (omega-6) were the most potent fatty acids. Omega-3 (n-3) and n-6 fatty acids contribute to fat regulation in the body, such as cholesterol-lowering. Algae DHA is also associated with positive effects for brain mass, memory, and learning. Chlorella sorokiniana could hence have potential health benefits not only through activation of PPAR-alpha and gamma, but also via its unique algae DHA constituents.17 In a double-blind clinical trial performed on 106 healthy, sedentary, overweight middle-aged and elderly individuals supplemented with 2.5 g of n-3s, researchers assessed whether n-3 PUFA supplementation could affect leukocyte telomere length, telomerase, and oxidative stress.18 The study concluded that telomere length increased with decreasing n-6:n-3 ratios.18 The data thus suggest that lower n-6:n-3 fat ratios can impact immune cell aging, an important pre-disease mechanism, suggesting the possibility for nutritional prevention and longevity.
Green Tea
Green tea (Camellia sinensis) is one of the most studied herbs for its panacea of benefits. And the research is moving into the field of gene expression. One recent study demonstrated that a single, high dose of green tea extract prevented the overproduction of nitric oxide (NO) in the heart cells of newborns.19 While NO is required for blood vessel relaxation, an overproduction of NO is related to conditions such as atherosclerosis, whereby inflammation in the arteries is associated with a dangerously high level of NO. In a follow-up study that looked at how green tea extract reverses overproduction of NO, it was observed that green tea’s antioxidant activity and PPAR-beta and delta activation were key to its therapeutic effect.20 Due to its ability to alter gene expression in this way, green tea has great potential in the treatment of heart and blood vessel disease.
Acetyl-L-Carnitine and Alpha-Lipoic Acid
Acetyl-L-carnitine and alpha-lipoic acid are 2 amino acid-like nutrients that have been shown in numerous trauma and concussion syndrome studies to work well symbiotically. One animal study demonstrated that defective fat and cholesterol metabolism is associated with low mitochondrial quality and activity in skeletal muscle of diabetic Goto-Kakizaki rats, thereby leading to metabolic syndrome, obesity, and diabetes.21 When diseased rats were treated with a combination of R-alpha-lipoic acid, acetyl-L-carnitine and 2 B vitamins (nicotinamide and biotin), the nutritional protocol effectively improved glucose tolerance, decreased the basal insulin secretion and the level of circulating free fatty acids, and maintained mitochondria health within the muscle itself. The nutrient protocol also significantly increased genetic tissue involved in lipid metabolism, including PPAR-alpha and delta activity of skeletal muscle mitochondria. All of these improvements on mitochondrial nutrients were shown to be comparable to that of the anti-diabetic drug, pioglitazone. In addition, the treatment with nutrients, unlike pioglitazone, did not cause body weight gain.21 Combined treatment of alpha-lipoic acid and acetyl-L-carnitine for a little as 24 hours significantly increased mitochondrial mass, expression of mitochondrial DNA, mitochondrial complexes, oxygen consumption, and fatty acid oxidation in fat cells.21 These changes were accompanied by an increase in expression of PPAR-gamma, PPAR-alpha and Cpt1a mRNA, as well as increased expression of PPAR-gamma co-activator 1 alpha (PPARgc1a), mitochondrial transcription factor A (Tfam), and nuclear respiratory factors 1 and 2 (Nrf1 and Nrf2).22
Epigenetics, Nutrigenomics, and Naturopathic Medicine
As naturopathic doctors, we have been schooled to innately and philosophically understand that the physical structure of the gene is not finite in predicting health and longevity. Hahnemmanian principles teach that the miasm is conditional for change through a gentle but accurate shift of the vital force. Chinese medicine teaches that an individual’s health potential is determined by the sum of acquired, parental, inherited, and wei qi. Naturopathic medicine connects the best of folklore and science as it considers the sum of all inherited and individual health influences. At this point in health research, novel nutritional and biochemical theories that challenge current medical and evolutionary paradigms are putting into perspective how these changes take place at the cellular and genetic levels. If anything, the fields of epigenetics and nutrigenomics insures naturopathic medicine’s course, as science continues to explain why naturopathic doctors successfully counsel their patients away from chronic illness with the use of food, herbs, and dietary supplements. It also sheds light on the fact that we may be inadvertently counseling patients towards a terminal youth or longevity syndrome prior to death. From the research cited above, nutrition and dietary supplements play a key role in the etiology of genetically-linked lifestyle conditions, from prevention to anti-aging and Alzheimer’s, to heart disease and cancer, making it plausible that our patients, led by us, will live until a healthy 120 years old.
 Millie Lytle, ND, MPH, CNS is an advocate in the natural health industry with over 20 years experience. She is a licensed Naturopathic Doctor (District of Columbia and Certified Nutrition Specialist living and practicing in New York and D.C.. She also has a Master in Public Health (Germany).
Millie Lytle, ND, MPH, CNS is an advocate in the natural health industry with over 20 years experience. She is a licensed Naturopathic Doctor (District of Columbia and Certified Nutrition Specialist living and practicing in New York and D.C.. She also has a Master in Public Health (Germany).
She is the founder the Eating for Meaning, a paradigm shift into individual nutrition teaching the consumer to eat according to their own wisdom. She is also the founder of Wellpath.me, a blockchain-based tech company focusing on self-help advocates and users of alternative medicine who’s mission is to reduce the top 6 chronic diseases by 50% over 25 years. She is a published researcher and author. She has a goal of living healthy until 120 years using Eating for Meaning at www.drmillie.com
References
- Chang J. The Aging Myth: Unlocking the Mysteries of Looking and Feeling Young. Aylesbury, UK: Aylesbury Publishing, LLC; 2011.
- The Free Dictionary by Farlex. Longevity Syndrome. http://medical-dictionary.thefreedictionary.com/Longevity+Syndrome. Accessed February 15, 2014.
- Lipton BH. The Biology of Belief: Unleashing the Power of Consciousness, Matter, & Miracles. Carlsbad, CA: Hay House; 2008.
- Balistreri C, Candore G, Accardi G, et al. Genetics of longevity data from the studies on Sicilian centenarians. Immun Ageing. 2012;9(1):8.
- Mitteldorf JJ. Telomere biology: Cancer firewall or aging clock? Biochemistry (Moscow).2013;78(9):1054-1060.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112-1120.
- Mitteldorf JJ. How does the body know how old it is? Introducing the epigenetic clock hypothesis. Biochemistry (Moscow). 2013;78(9):1048-1053.
- Accardi G, Virruso C, Balistreri CR, et al. SHIP2: a “NEW” insulin pathway target for ageing research. Rejuvenation Res. 2013 Dec 9. [Epub ahead of print]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409-35.
- Stienstra R, Duval C, Müller M, Kersten S. PPARs, Obesity, and Inflammation. PPAR Res. 2007;2007:95974.
- Youssef J, Badr MZ. PPARs: history and advances. Methods Mol Biol. 2013;952:1-6.
- Wakeling LA, Ions LJ, Ford D. Could Sirt1-mediated epigenetic effects contribute to the longevity response to dietary restriction and be mimicked by other dietary interventions? Age (Dordr). 2009;31(4):327-341.
- Ford D, Ions LJ, Alatawi F, Wakeling LA. The potential role of epigenetic responses to diet in ageing. Proc Nutr Soc. 2011;70(3):374-384.
- Allard JS, Perez E, Zou S, De Cabo R. Dietary activators of Sirt1. Mol Cell Endocrinol. 2009;299(1):58-63.
- Rimando AM, Nagmani R, Feller DR, Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem. 2005;53(9):3403-3407.
- Chang J, Rimando A, Pallas M, et al. Neurobiol Aging. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. 2012;33(9):2062-2071.
- Chou YC, Prakash E, Huang CF, et al. Bioassay-guided purification and identification of PPARalpha/gamma agonists from Chlorella sorokiniana. Phytother Res. 2008;22(5):605-613.
- Kiecolt-Glaser JK, Epel ES, Belury MA, et al. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain Behav Immun. 2013;28:16-24.
- Agnetti G1, Bordoni A, Angeloni C, et al. Green tea modulation of inducible nitric oxide synthase in hypoxic/reoxygenated cardiomyocytes. Biochimie. 2005;87(5):457-460.
- Danesi F, Di Nunzio M, Boschetti E, Bordoni A. Green tea extract selectively activates peroxisome proliferator-activated receptor beta/delta in cultured cardiomyocytes. Br J Nutr. 2009;101(12):1736-1739.
- Shen W, Hao J, Tian C, et al. A combination of nutriments improves mitochondrial biogenesis and function in skeletal muscle of type 2 diabetic Goto-Kakizaki rats. PLoS One. 2008;3(6):e2328.
- Shen W, Liu K, Tian C, et al. R-alpha-lipoic acid and acetyl-L-carnitine complementarily promote mitochondrial biogenesis in murine 3T3-L1 adipocytes. Diabetologia. 2008;51(1):165-174.