Jillian Stansbury, ND
Dopamine is increasingly being recognized as a key regulatory neurohormone in the hypothalamus and the pituitary. Dopamine and L-Dopa inhibit the release of some pituitary hormones. Most notable are prolactin and luteinizing hormone (LH); possibly inhibited is thyroid-stimulating hormone (TSH) and, in some cases, so is growth hormone (GH). Insulin response and complex feedback loops between the endocrine glands are known to underlie many cases of infertility and polycystic ovarian syndrome (PCOS); these also underlie many complex thyroxine (T4) and insulin resistance cases.
Current research is investigating how dopamine and dopamine agonists may affect GH and prolactin secretion, as well as how they may affect insulin, follicle-stimulating hormone (FSH), LH, and other hormones. Since PCOS involves alterations in all of these hormones, the role of dopamine is pertinent to PCOS pathophysiology.
Dopamine and Prolactin
Dopamine acts as a co-regulator of prolactin, GH, LH, and possibly other gonadotropins and thyrotrophins, reducing the amounts released from the pituitary. Since dopamine inhibits the release of prolactin and LH, low levels of dopamine may lead to excessive release of prolactin from the pituitary (Prelevic GM et al., 1987; Zironi C et al., 1991). Hyperprolactinemia can cause infertility, amenorrhea, galactorrhea and mastalgia, and is also associated with PCOS. Many infertile women with PCOS will improve their chances of conception by increasing dopamine levels with dopamine agonists and anti-estrogens (Harrison et al., 1993). Prolactin may also alter the ratio of testosterone to dihydrotestosterone and promote aromatization into estrogen. Many women with elevated prolactin, such as in PCOS, have low progesterone relative to estrogen and testosterone. These women may benefit from progesterone and anti-estrogens, and also weak phytoestrogens and aromatase inhibitors.
Conversely, elevations in dopamine levels will lead to very low levels of prolactin and LH. Clinical trials have shown intravenous administration of dopamine to men and women to result in decreased prolactin and gonadotropins in free circulation (Speroff et al., 1983).
Some of the dopamine agonist drugs, such as cabergoline, are noted to normalize androgen levels in women with PCOS in addition to reducing hyperprolactinemia (Paoletti et al. 1996). The use of dopamine-promoting herbs, such as Melissa citronelle (lemon balm), Cimicifuga racemosa (black cohosh) and Vitex agnus castus (chaste tree berry) may reduce hyperprolactinemia and androgen excess. Because gamma-aminobutyric acid (GABA) agonists may in turn promote dopamine, many botanical GABA agonists may also help regulate prolactin. Opiates, in turn, promote GABA and therefore dopamine levels as well. Vitex has also been shown to promote opioid activity in the brain.
GABA Agonists and Prolactin
The pharmaceutical GABA (B) agonist, baclofen, has been shown to inhibit secretion of prolactin (Lux-Lantos V et al., 1992). Since Hypericum perforatum (St. John’s wort) increases dopamine by reuptake inhibition as well as by acting as a GABA agonist, this botanical agent may benefit those with PCOS. Other botanicals noted to bind GABA receptors and act as agonists include Leonurus cardiaca (motherwort), Valeriana officinalis (valerian), Withania somnifera (Ashwagandha), Melissa, Piper methysticum (kava), and Tilia (linden). L-Theanine, found in Camellia sinensis (green tea), may also bind GABA receptors.
Effect of Estrogen on the Thyroid
The fact that thyroid disease is 10 times more common in women compared to men suggests an interaction between estrogen and the thyroid. For example, estrogen increases hepatic synthesis and release of thyroid-binding globulins. Estrogen may also increase T4 and T3 uptake in cases of hyperthyroidism, but not in euthyroid subjects. In fact, oral contraceptives and pregnancy are both noted to resolve some cases of Graves’ disease (Wiegratz et al., 2003). Elevated estrogen, such as what commonly occurs with estrogen dominance, leads to increased uptake and binding of thyroid hormones. This can lead to overstimulation of the thyroid gland and thyroxine resistance over time. Thus, high estrogen levels can both exacerbate a hyperfunctioning thyroid gland, as well as contribute to further downregulation in an unresponsive thyroid gland. Human chorionic gonadotropin is reported to support weight loss by improving general metabolism. It may also have to do with improving the response of thyroid cells to TSH and improving cellular metabolism.
Anything that can reduce the estrogen load in the body may help both PCOS and ovarian stimulation, as well as improve several types of thyroid dysfunction. Estrogen dominance may be balanced by the use of alterative herbs such as Taraxicum officinalis (dandelion), Mahonia (Oregon grape), Curcuma longa (turmeric), and others by helping the liver conjugate and excrete these hormones. Aromatase inhibitors would also be helpful in reducing hyperestrogenism. Aromatase inhibitors are well known to reduce the synthesis of estrogen from progesterone and testosterone in peripheral adipose tissues. Less well known is that aromatase inhibitors may also act on thyroid hormones. The administration of aromatase inhibitors to poultry causes an increase in T3 and T4. Urtica dioica (nettles), Serenoa repens (saw palmetto), and Prunus africana (pygeum africanum) may all act as aromatase inhibitors as do the cruciferous vegetables and the indoles such as (diindolylmethane) DIM.
THE THYROID GLAND AND PCOS
Many women with PCOS also have either concomitant hypothyroidism, or all the classic symptoms with normal thyroid hormone levels. Improving adrenal and thyroid activity may restore menses in amenorrheic women.
Stimulants such as the methylxanthine-containing plants coffee and green tea will also promote dopaminergic activity in the brain. Coleus forskohlii is a traditional herb used for hypothyroidism in Ayurvedic medicine. Forskolin, a diterpene in Coleus may promote adenylate cyclase, thereby increasing intracellular concentrations of cAMP. This action may have a general upregulating effect on tissues particularly responsive to cAMP such as the liver, adrenals, and thyroid (Ding and Staudinger, 2005). Coleus may therefore be an appropriate synergist in formulas for hypothyroidism. Other stimulants such as Illicium verum (star anise) and C. sinensis may also be useful to complement the dopamine- and GABA-enhancing herbs for women with lethargy, weight loss difficulty, and low thyroid function.
THYROXINE RESISTANCE
Like insulin resistance, some cases of hypothyroidism may result from impaired tissue response to thyroxine. The thyroid gland becomes downregulated and T4 and T3 levels decline due to poor response to TSH. Thus, TSH increases as the brain attempts to elevate deficient thyroid hormone levels, creating an unproductive cycle. The cause of this is not well understood. A genetic mutation in the sequence coding for the thyroid hormone receptor is being investigated by some researchers, but other researchers feel the cause is multifactorial, as is the case with Syndrome X and insulin resistance. Melissa extracts have been noted to bind thyrotropin, thereby reducing excessive stimulation of the thyroid leading to resistance (Sourgens et al., 1982).
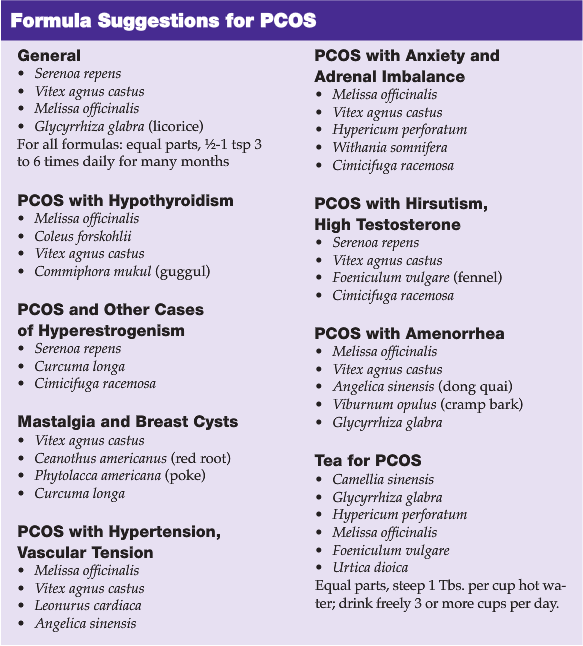
BOTANICALS FOR PCOS
As has been discussed, agents that promote dopamine, reduce prolactin, bind GABA receptors, and enhance thyroid activity can help women with PCOS via a variety of mechanisms. Botanicals particularly useful for PCOS are effective via a number of these mechanisms simultaneously. I will focus on reviewing Melissa, but will mention briefly other important herbs.
Cimicifuga racemosa is known to affect dopamine and the neuroendocrine system benefiting both mood disorders and hormonal imbalances. Cimicifuga is noted to exert activity at both estrogen receptors locally (Jarry et al., 1985; Jarry and Harnischfeger, 1985) and in the pituitary at the level of the neuroendocrine system (Jarry et al., 1995; Duker et al., 1991). Cimicifuga contains isoflavones that are believed to exert amphoteric actions at estrogen receptors.
Recent research has revealed a new group of guanidine alkaloids in Cimicifuga, one of them named dopargine and said to be a derivative of dopamine. These alkaloids are being credited with neurotransmitter effects including serotonergic actions (Godecke T et al., 2009). Cimicifuga has been reported to displace estradiol from docking at estrogen receptors, as well as to bind dopamine receptors and promote dopaminergic neurotransmission (Jarry et al., 2003).
Leonurus cardiaca may affect mood and act as a nervine via dopamine activity, and help reduce excess TSH via a dopamine mechanism. Leonurus may also have neuroendocrine effects, via dopamine as well as numerous vascular effects. Because both the uterine muscle and the smooth muscle cells of the vasculature possess beta receptors, plant-based “beta blockers” like Leonurus may be appropriate for both tonifying the uterus and reducing vasoconstriction in cases of hypertension (Kong YC et al., 1976; Zou QZ et al., 1989; Bird and Wingham, 1979, Lee et al., 1991). In addition to simply relaxing uterine muscle, Leonurus has been reported to inhibit the growth of uterine fibroids in mice, presumably due to other endocrine effects that may reduce hyperestrogenism. Researchers attempted to isolate the constituent responsible for the antineoplastic and antifibroid effects in mice, but found the synergistic action of all the components to be most effective (Nagasawa et al.,1992).
Vitex agnus castus is believed to exert an overall progesteronic influence making it useful for numerous conditions associated with estrogen dominance, including adolescent acne, PMS, breast pain and cysts, uterine fibroids, PCOS, and climacteric disorders. Vitex is reported to have a dopaminergic effect and, in turn, have a prolactin-suppresive effect. Mastalgia can result from hyperprolactinemia and Vitex is reported to be an effective therapy for mastalgia (Carmichael, 2008). Since dopamine also controls the release of LH which promotes testosterone production in men and testosterone and progesterone in women, Vitex may improve acne in adolescent males and reduce the elevated testosterone common in women with PCOS. In studies, Vitex has been shown to reduce LH and testosterone elevations in men (Nasri et al., 2007). Vitex has also been shown to reduce prolactin in women with high levels via dopaminergic effects (Wuttke et al., 2003).
The search for the chemical identity of the dopaminergic compounds has resulted in isolation of a number of diterpenes of which some clerodadienols were most important for the prolactin-suppressive effects. Researchers reported these Vitex constituents to reduce prolactin release as powerfully as dopamine itself.
Hypericum perforatum has an inhibitory effect on the neuronal reuptake of dopamine, in addition to serotonin, noradrenaline, GABA and L-glutamate. It is only the constituent hyperforin and its structural analogue adhyperforin that inhibit the reuptake. (Muller, 2003; Tadros et al., 2009). However, instead of acting as a competitive inhibitor at the transmitter binding sites of the transporter proteins, hyperforin affects the sodium gradient leading to an inhibition of uptake. Inhibition of neurotransmitter reuptake occurs due to affecting sodium gradients on neuronal cells. Due to dopaminergic effects, Hypericum might be considered for breast pain, climacteric disorders, PCOS, and other endocrine imbalances, in addition to the commonly discussed indications for anxiety and depression.
Prolactin may also play a role in immune regulation. Both Echinacea purpurea (purple coneflower) and Hypericum are reported to have dopaminergic activity. Researchers have proposed that these plants may improve immune response via a dopaminergic mechanism, affecting prolactin, and in turn affecting immune response (Di Carlo et al., 2005).
Melissa citronelle may inhibit prolactin via dopamine activity which would be an explanation for the folkloric use of lemon balm for depression. Melissa has been referred to as “the gladdening herb” in folklore, and modern research has found mood elevation to occur via a number of neurotransmitter effects. However, in addition to being a nervine, Melissa has had a long-standing reputation as an estrogen and thyroid hormone effector. Some research suggests that Melissa may affect the endocrine system via activity on the neurotransmitter regulators of gonadotrophins and thyrotropins. Dopamine may regulate prolactin and gonadotropins, and Melissa may exert dopaminergic effects thereby having both mood elevating and hormonal effects (Sourgens et al., 1982).
In addition to promoting dopamine, Melissa is noted to have activity at GABA receptors which would also provide anxiolytic activity. Activity at muscarinic and nicotinic acetylcholine receptors has also been demostrated. Cholinesterase inhibition displayed by Melissa may be another mood and cognition enhancing mechanism (Awad et al., 2009; Kennedy et al., 2003).
Melissa has been reported to have general thyroid-inhibiting effects (Auf’mkolk, Amir et al., 1985). The use of Melissa in the treatment of mood disorders has not been reported to induce hyperthyroidism in euthyroid individuals. Modern research has shown Melissa to reduce TSH when excessive via neuroendocrine effects on neurotransmitters, effects on thyroid gland cells, as well as direct effects on TSH (Auf’mkolk, Ingbar et al., 1985). One mechanism may be via binding directly to TSH and reducing its activity (Sourgens et al., 1982; Santini et al., 2003). Another mechanism may be a reduction in the response of thyroid gland cells to TSH itself, or binding by thyroid auto antibodies in the case of Graves’ disease (Santini et al., 2003; Auf’mkolk, Amir et al., 1985). Molecular constituents in Melissa may form loosely-bound adducts with TSH affecting binding to cellular receptors. By reducing TSH stimulation of the thyroid, Melissa may also help thyroid tissue that has become resistant to TSH to recover.
Melissa may have anodyne effects due to an influence on neurotransmission (Guginski et al., 2009). Rosmarinic acid in Melissa is reported to contribute to the analgesic effects. One clinical trial for pain in human subjects reported efficacy without significant side effects (Melzer et al., 2009). Extracts of the whole plant are reported to be potent inhibitors of GABA transaminase, the enzyme that degrades GABA, thereby increasing blood levels of this primary relaxing neurotransmitter (Awad et al., 2009). Rosmarinic acid and the triterpenoids ursolic and oleanolic acids were reported to be among the identifiable GABA agonists. Luteolin, a flavonoid in Melissa was shown in animal studies to have anxiolytic effects, however the researchers reported that luteolin did not possess GABA (A) receptor activity, indicating additional mechanisms of action yet to be found (Raines et al., 2009). Melissa may slow the heart rate due to gabaminergic effects (Gazola et al., 2004).
Due to these numerous effects on the thyroid, Melissa may be appropriate for hyperthyroidism as well as thyroxine resistance causing symptoms of low thyroid. Due to effects on both dopamine and GABA, which promotes dopamine, Melissa has anxiolytic and anti-depressant activity, as well as an ability to reduce prolactinemia and all of the amenorrheic, PCOS, and complex insulin resistant endocrine imbalances that accompany it.
 Jillian Stansbury, ND has practiced in SW Washington for nearly 20 years, specializing in women’s health, mental health and chronic disease. She holds undergraduate degrees in medical illus-tration and medical assisting, and gradu-ated with honors in both programs. Dr. Stansbury also chairs the botanical medi-cine program at NCNM and teaches the core botanical curricula, a position she has held for over 18 years. In addition, Dr. Stansbury also writes and serves as a medical editor for numerous professional journals and lay publications, plus teaches natural products chemistry and herbal medicine around the country. At present she is working to set up a humanitarian service organization in Peru and studying South American ethnobotany. She is the mother of two adult children, and her hobbies include art, music, gardening, camping and international travel.
Jillian Stansbury, ND has practiced in SW Washington for nearly 20 years, specializing in women’s health, mental health and chronic disease. She holds undergraduate degrees in medical illus-tration and medical assisting, and gradu-ated with honors in both programs. Dr. Stansbury also chairs the botanical medi-cine program at NCNM and teaches the core botanical curricula, a position she has held for over 18 years. In addition, Dr. Stansbury also writes and serves as a medical editor for numerous professional journals and lay publications, plus teaches natural products chemistry and herbal medicine around the country. At present she is working to set up a humanitarian service organization in Peru and studying South American ethnobotany. She is the mother of two adult children, and her hobbies include art, music, gardening, camping and international travel.
References
Auf’mkolk M, Amir SM et al. The active principles of plant extracts with antithyrotropic activity: oxidation products of derivatives of 3,4-dihydroxycinnamic acid. Endocrinology. 1985;116(5):1677-1686.
Auf’mkolk M, Ingbar JC et al. Extracts and auto-oxidized constituents of certain plants inhibit the receptor-binding and the biological activity of Graves’ immunoglobulins. Endocrinology. 1985;116(5):1687-1693.
Awad R et al. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother Res. 2009 Jan 22. [Epub ahead of print].
Bird GW, Wingham J. Anti-Cad lectin from the seeds of Leonurus cardiaca. Clin Lab Haematol. 1979;1(1):57-59.
Carmichael AR. Can vitex agnus castus be used for the treatment of mastalgia? What is the current evidence? Evid Based Complement Alternat Med. 2008;5(3):247-250.
Darmstadt: Steinkopff-Verlag; 1995:99-112. Treatment of menopausal symptoms with extracts of Cimicifuga racemosa: in vivo and in vitro evidence of estrogenic activity. In: Loew D, Reitbrock N, eds. Phytopharmaka in Forschung und klinischer Anwendung. Jarry H, Gorkow C, Wuttke W.
Di Carlo G et al. Effect on prolactin secretion of Echinacea purpurea, hypericum perforatum and Eleutherococcus senticosus. Phytomedicine. 2005;12(9):644-647.
Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase A signal transduction pathway. J Pharmacol Exp Ther. 2005;312(2):849-856.
Duker EM et al. Effects of extracts from Cimicifuga racemosa on gonadotropin release in menopausal women and ovariectomized rats. Planta Med. 1991;57(5):420-424.
Gazola R et al. Lippia alba, Melissa officinalis and Cymbopogon citratus: effects of the aqueous extracts on the isolated hearts of rats. Pharmacol Res. 2004;50(5):477-480.
Godecke T et al. Guanidine alkaloids and Pictet-Spengler adducts from black cohosh (Cimicifuga racemosa). J Nat Prod. 2009;72(3):433–437.
Guginski G et al. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol Biochem Behav. 2009;93(1):10-16.
Harrison RF et al. Can women with gonadotropin levels diagnostic of polycystic ovarian syndrome benefit from therapy with dopamine agonists? Ann NY Acad Sci. 1993;May 28;687:272-279.
Jarry H et al. Studies on the endocrine effects of the contents of Cimicifuga racemosa 2. In vitro binding of compounds to estrogen receptors. Planta Med. 1985;51(4):316-319.
Jarry H et al. In vitro effects of the Cimicifuga racemosa extract BNO 1055. Maturitas. 2003;44 Suppl 1:S31-38.
Jarry H, Harnischfeger G. Studies on the endocrine effects of the contents of Cimicifuga racemosa. Planta Med. 1985;51(1):46-49.
Kennedy DO et al. Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology. 2003;28(10):1871-1881.
Kong YC et al. Isolation of the uterotonic principle from Leonurus artemisia, the Chinese motherwort. Am J Chin Med. 1976;4(4):373-382.
Lee CM et al. Prehispanolone, a novel platelet activating factor receptor antagonist from Leonurus heterophyllus. Br J Pharmacol. 1991;103(3):1719-1724.
Lux-Lantos V et al. Activation of GABA B receptors in the anterior pituitary inhibits prolactin and luteinizing hormone secretion. Neuroendocrinology. 1992;56(5):687-693.
Melzer J et al. Fixed herbal drug combination with and without butterbur (Ze 185) for the treatment of patients with somatoform disorders: randomized, placebo-controlled pharmaco-clinical trial. Phytother Res. 2009 Mar 9. [Epub ahead of print].
Muller WE. Current St John’s wort research from mode of action to clinical efficacy. Pharmacol Res. 2003;47(2):101-109.
Nagasawa H et al. Further study on the effects of motherwort (Leonurus sibiricus L) on preneoplastic and neoplastic mammary gland growth in multiparous GR/A mice. Anticancer Res. 1992;12(1):141-143.
Nasri S et al. The effects of Vitex agnus castus extract and its interaction with dopaminergic system on LH and testosterone in male mice. Pak J Biol Sci. 2007;10(14):2300-2307.
Paoletti AM et al. The chronic administration of cabergoline normalizes androgen secretion and improves menstrual cyclicity in women with polycystic ovary syndrome. Fertil Steril. 1996;66(4):527-532.
Prelevic GM et al. Acute effects of L-dopa and bromocriptine on serum PRL, LH and FSH levels in patients with hyperprolactinemic and normoprolactinemic polycystic ovary syndrome. J Endocrinol Invest. 1987;10(4):389-395.
Raines T et al. Investigation of the anxiolytic effects of luteolin, a lemon balm flavonoid in the male Sprague-Dawley rat. AANA J. 2009;77(1):33-36
Santini F et al. In vitro assay of thyroid disruptors affecting TSH-stimulated adenylate cyclase activity. J Endocrinol Invest. 2003;26(10):950-955.
Sourgens H et al. Antihormonal effects of plant extracts. TSH- and prolactin-supressing properties of Lithospermum officinale and other plants. Planta Med. 1982;45(2):78-86.
Speroff, Glass, and Kase, Clinical Gynecologic Endocrinology and Infertility 3rd Edition,Williams and Wilkins pg 49 1983.
Tadros MG et al. Involvement of serotoninergic 5-HT1A/2A, alpha-adrenergic and dopaminergic D1 receptors in St. John’s wort-induced prepulse inhibition deficit: a possible role of hyperforin. Behav Brain Res. 2009;199(2):334-339.
Wiegratz I et al. Effect of four oral contraceptives on thyroid hormones, adrenal and blood pressure parameters. Contraception. 2003;67(5):361-366.
Wuttke W et al. Chaste tree (Vitex agnus-castus)–pharmacology and clinical indications. Phytomedicine. 2003;10(4):348-357.
Zironi C et al. Evaluation of dopaminergic activity of the hypothalamus in patients with polycystic ovarian syndrome. Minerva Ginecol. 1991;43(10):443-447.
Zou QZ et al. Effects of motherwort on blood hyperviscosity. Am J Chin Med. 1989;17(1-2):65-70.