JAKE F. FELICE, ND, LMP
This article discusses a newly discovered apoptogenic feature of the endocannabinoid system (ECS), known as retrograde transmission. It also discusses important influences of the ECS on neural plasticity, as well as connective tissue modulation of the nervous system via the ECS. For readers interested in detailed information about the ECS, please refer to one of my previous articles.1
In certain illnesses, hyperactivity of the nervous system is a contributing feature to pathology. This includes the case of seizures in epilepsy where “electrical storms” rage in the brain and too many neurons are pathologically overstimulated. This can be a consequence of inadequate inhibition. When inhibitory mechanisms of the nervous system become impaired, “dis-inhibition disorders” such as hyperactivity, impulse behaviors, anxiety, stress, seizures, central sensitization, and pain exacerbation can occur. Via retrograde signaling, the ECS allows neurons on the receiving end of overactive impulses to call a “time-out” by sending signals upstream to quiet the overactive inputs from the nervous system.2 This is thought to be one of the ways the ECS protects the nervous system from hyperactivity3: it gives overstimulated presynaptic neurons and their neural circuitry a modulatory mechanism to control excessive pathologic inputs.4,5
Retrograde Transmission: An Overview
Prior to the discovery of retrograde signaling via the ECS, all neural impulses were assumed to be unidirectional. In this conventional model, neurotransmitters are released from the presynaptic neuron and then diffuse to the postsynaptic neuron where they bind to receptors, thereby generating nerve impulses that convey biologic information.
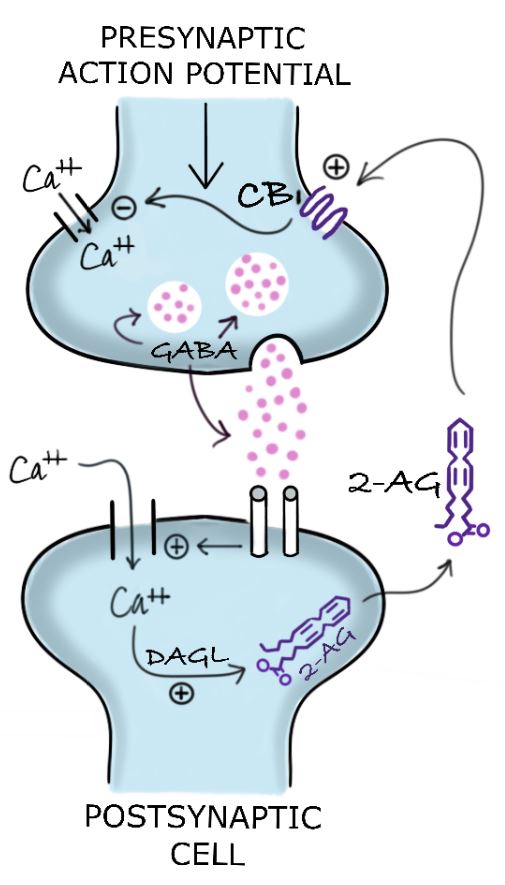
With the discovery of retrograde transmission via the ECS, this conventional model of neuronal function was turned on its head. Animal research has shown that diffusible endocannabinoids are released on demand, in an activity-dependent manner, from lipid precursors in postsynaptic neurons, and then diffuse “backwards” across the synapse to activate cannabinoid (CB1) receptors on the presynaptic neuron.6 This activation of presynaptic CB1 receptors by post-synaptically discharged endocannabinoids results in decreased presynaptic neurotransmitter release.7,8 This retrograde feature of the ECS allows postsynaptic neurons to control the strength of their inputs and reduce overstimulation from presynaptic neurons. This process represents a powerful physiologic negative feedback system9 and is a key element in the ECS’ homeostatic regulation of overactive nerve transmission.
Examples of neurotransmitters whose release is inhibited by the activation of CB1 receptors in the presynapse include GABA (inhibitory) and glutamate (excitatory).8 The ECS’ temporary reduction of the release of excitatory neurotransmitters such as glutamate is designated “depolarization-induced suppression of excitation” (Figure 1). ECS inhibition of the release of inhibitory neurotransmitters such as GABA is known as “depolarization-induced suppression of inhibition” (Figure 2).
The idea that a postsynaptic neuron influences or alters neurotransmitter release from its presynaptic counterpart represents a huge paradigm shift in our understanding of biologic information exchange.
Figure 1. Depolarization-Induced Suppression of Excitation
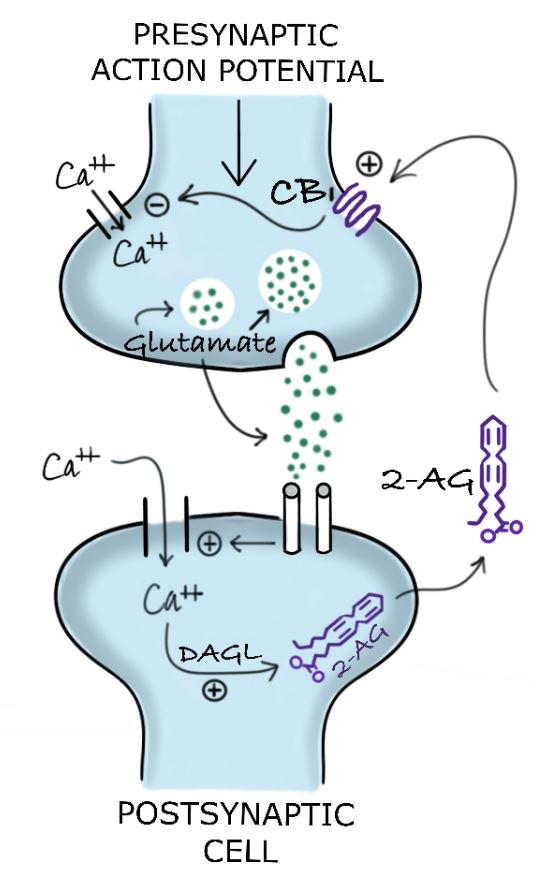
Figure 2. Depolarization-Induced Suppression of Inhibition
Clinical Implications
In traumatic brain injury (TBI), mounting data suggest that endocannabinoids provide much of their neuroprotective effects through retrograde inhibition.10 Following adverse brain events such as TBI, increased synthesis of the endocannabinoid 2-AG serves as a homeostatic regulator to limit brain damage during hyperactive events,11 where endocannabinoids can diffuse backwards across the synaptic cleft and facilitate a quieting of the neural circuitry via the postsynaptic neuron. Reactive oxygen species, inflammatory cytokines, and blood-brain-barrier permeability are reduced in the process.10,11 Intriguingly, animal studies demonstrate that accumulated 2-AG begins providing neuroprotection via reduction of proinflammatory cytokines within as little as 2-4 hours of brain injury.11
Retrograde inhibition also works on pain pathways. Animal research has shown that when a pain signal is extreme or overwhelming, retrograde inhibition uses endocannabinoid signaling to slow or reduce the nociceptive impulses coming from the site of the injury, thereby reducing the signals moving towards the cortex.12
The downregulation of the ECS by stress cascades may also be of particular clinical interest, as the inhibitory effects of stress on the ECS may represent a type of disinhibition disorder.13,14
ECS & Synaptic Regulation by Connective Tissue
In traditional neural communication models, biological information transfer was thought to be exclusively directed at the chemical synapses between individual neurons. Connective tissue cells, such as glia and astrocytes, were only thought to provide structural support to nerve cells. However, it is now believed that bidirectional communication occurs between neurons and connective tissue cells in various modes of synaptic transmission.15
The temporal window through which the interstitium modifies neuronal activity via the ECS may significantly influence its effects.16 In seizures, for example, persistent changes in neuronal excitability can induce changes in retrograde signaling, with the potential to enhance homeostatic responses.16
Studies show that nearly half of all synapses involve direct connections between neurons and astrocytes in a manner that influences and promotes biologic communication.15,17 In this new model of the synapse, astrocytes interface with both the dendrites and axons of post- and presynaptic neurons, where they monitor neurotransmitter communication in the extracellular fluid of the synapse.15
Although astrocytes do not directly stimulate action potentials like neurons do, their method of excitation involves creating a transient increase of intracellular calcium, which then triggers a release of neurotransmitters known as “gliotransmitters.”15,18-20
It is postulated that astrocyte signaling pathways act widely and not just locally and that each individual astrocyte has the potential to connect with and influence an enormous number of neurons, thus influencing various synapses at a distance.15,21 The spread of endocannabinoids within the extracellular space of a neural synapse is spatially limited. However, the potentiation mediated by astrocytes gives the ECS the ability to spread biologic information more widely. This is because astrocytes can release glutamate from their internal stores at an area far from the original synapse.22 It is now hypothesized that these connective-tissue-based signaling pathways provide catalysts for the self-repair of damaged synapses.15,23,24
Thus, the regulation of communication via astrocytes’ spatiotemporal control of retrograde transmission gives the interstitium a previously unrecognized degree of control over neural signaling pathways. It now appears that self-diagnostic and self-repair properties of the ECS are being guided, at least partially, by connective tissue (interstitium) elements.15 This is a profound development in our understanding of the ECS’ function, and it provides new avenues for future research and development.
Neural Plasticity & the ECS
Recent research also indicates that in addition to its many functions, the ECS, via endocannabinoids, influences neuroplasticity in numerous regions of the central nervous system.8,22 Here, retrograde ECS signaling is essential for short-term and long-term plasticity at both excitatory and inhibitory synapses, where the ECS influences various neurologic functions, including learning and memory.7 CB1-knockout mice, for example, exhibit multiple defects in synaptic activity and plasticity, as well as behavioral abnormalities.7
Conclusion
The ECS and cannabinoids profoundly influence neurotransmission at the neural synapse via retrograde signaling. Additionally, the ECS, via astrocytes and glial cells, gives the connective tissue matrix a way to broadly modify neural transmission and information exchange at the synapse.
Cannabis and the ECS can alleviate chronic pain by slowing nociceptive signaling, as well as prevent glutamate toxicity and seizures via retrograde inhibition. They also help stimulate repair and reduce injury by decreasing inflammatory cytokines locally, on spinal sites, and in the brain.
ECS elements are also significantly involved in neural plasticity. Interestingly, the degree of presynaptic retrograde release is dependent on the state of health of the organism.16 Whether variations in retrograde signaling are the effect or cause of pathology is yet unclear and should be addressed by future research in the field.
References:
- Felice JF. The Endocannabinoid System: Self-Regulating Harm Protection. NDNR. 2018;14(7):1,3. Available at: https://ndnr.com/pain-medicine/the-endocannabinoid-system-self-regulating-harm-protection/. Accessed May 23, 2021.
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95(14):8268-8273.
- McPartland JM. The endocannabinoid system: an osteopathic perspective. J Am Osteopath Assoc. 2008;108(10):586-600.
- Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neurosci Biobehav Rev. 2016;64:359-381.
- Panikashvili D, Simeonidou C, Ben-Shabat S, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413(6855):527-531.
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog Neurobiol. 2002;68(4):247-286.
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13(2):127-137.
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27(5):1211-1219.
- Mackie K. Mechanisms of CB1 receptor signaling: Endocannabinoid modulation of synaptic strength. Int J Obes (Lond). 2006;30 Suppl 1:S19-S23.
- Mechoulam R, Panikashvili D, Shohami E. Cannabinoids and brain injury: Therapeutic implications. Trends Mol Med. 2002;8(2):58-61.
- Mechoulam R, Shohami E. Endocannabinoids and traumatic brain injury. Mol Neurobiol. 2007;36(1):68-74.
- Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2012;8(6):403-421.
- Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 2014;29:1-8.
- Felice JF. The ECS-Adrenal-Stress Axis. NDNR. 2021;17(2):21. Available at: https://ndnr.com/neurology/the-ecs-adrenal-stress-axis/. Accessed May 24, 2021.
- Wade JJ, McDaid LJ, Harkin J, et al. Exploring retrograde signaling via astrocytes as a mechanism for self repair. In: The 2011 International Joint Conference on Neural Networks. 2011:3149-3155. Available at: https://tinyurl.com/4338zr74. Accessed May 23, 2021.
- Iremonger KJ, Wamsteeker Cusulin JI, Bains JS. Changing the tune: Plasticity and adaptation of retrograde signals. Trends Neurosci. 2013;36(8):471-479.
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208-215.
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8(3):429-440.
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16(16):5073-5081.
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6(8):626-640.
- Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68(1):113-126.
- Ohno-Shosaku T, Tanimura A, Hashimotodani Y, Kano M. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 2012;18(2):119-132.
- Nadkarni S, Jung P. Dressed neurons: Modeling neural-glial interactions. Phys Biol. 2004;1(1-2):35-41.
- Nadkarni S, Jung P. Modeling synaptic transmission of the tripartite synapse. Phys Biol. 2007;4(1):1-9.

Jake F. Felice, ND, LMP is a cannabis author, clinician, educator, and consultant whose vision is to advance the science and practical application of cannabis for medical and recreational markets around the world. Dr Felice provides world-class educational experiences by speaking authentically about hemp and cannabis. He consults with healthcare providers, industry, and the general public. His Category 1 CME courses for doctors, nurses, and pharmacists has been translated into 4 languages. Dr Felice is the founder of Cannabis Matrix Consulting, LLC, and he maintains a regular cannabis blog at drjakefelice.com.


