Non-Small Cell Lung Cancer (NSCLC) & Naturopathic Oncology
Vis Medicatrix Naturae
Maximizing Outcomes Using KNOWoncology.org – Part 1
Jen Green, ND, FABNO
Dugald Seely, ND, MSc, FABNO
Heather Wright, ND, FABNO
Naturopathic oncology combines current best evidence, clinical experience, and patient preferences. This article is the first part of a 2-part article demonstrating how naturopathic doctors can apply research, individualization, and experience to enhance standard-of-care treatment in general, as well as specifically for advanced Non-Small Cell Lung Cancer (NSCLC). This article also highlights a dynamic clinical tool called KNOW (the Knowledge in Naturopathic Oncology Website), which enables naturopathic doctors to quickly access relevant research in naturopathic oncology. In Part 2 we will present 2 cases of patients living with NSCLC, in order to demonstrate how this information can be put into practice.
KNOWoncology.org
KNOW (accessible at www.KNOWoncology.org) is a database of clinically relevant research supported by the Oncology Association of Naturopathic Physicians. It is regularly updated by a medical librarian through systematic searches of Medline and EMBASE for human studies related to natural therapies and cancer care. Following completion of each search, 2 people screen the records for relevant citations. Then full text articles are sourced and summarized to report study design, population details, intervention details, assessed outcomes, and adverse events. KNOW research assistants also tag each summary according to type of natural therapy, conventional therapy, and side effects of conventional care (eg, neuropathy, nausea). This allows each summarized study to be fully searchable within the KNOW website. KNOW is up to date and complete for breast cancer, neuropathy, curcumin, and lung cancer topics (and subtopics) from 2010-2016. We are currently working on summarizing studies from all tumor types for the years 2010-2017. KNOW is a research project that forwards the field of naturopathic oncology and is designed to power articles, systematic reviews, and other evidence-based content. KNOW empowers clinicians and patients with current best evidence. To gain access, simply become an oncANP member at www.oncanp.org.
Non-Small Cell Lung Cancer
While early NSCLC is treatable, stage III and IV lung cancer are challenging and can have very poor 5-year survival.1 Treatment for stage III NSCLC typically includes some combination of chemotherapy, radiation, and/or surgery. If cancer recurs or progresses on these treatments, or if someone has more extensive stage IV disease, then targeted therapy or immunotherapy is used.
Lung tumor tissue should get tested for common gene mutations, such as in the EGFR, ALK, ROS1 and PD-L1 genes. If one of these genes is mutated in stage IV NSCLC, first-line treatment would likely be a targeted therapy. For tumors that have the ALK gene mutation, alectinib,2 crizotinib, or ceritinib can be used. Other ALK inhibitors, such as alectinib or brigatinib, can be used if crizotinib stops working or is not well tolerated. For people whose cancers are associated with changes in the EGFR gene, the anti-EGFR drugs – erlotinib, gefitinib, or afatinib – can be used. For people whose cancers have changes in the ROS1 gene, an ALK inhibitor such as crizotinib might be used. And for people with the PD-L1 protein, immunotherapy with pembrolizumab or nivolumab is an option.3
Goals of a supportive naturopathic oncology plan include: maintaining weight, improving quality of life and minimizing dose-limiting side effects (ie, a side effect requiring oncologists to reduce or delay treatment). The major goals include better patient tolerance of treatment and improved survivorship. In lung cancer, dose-limiting side effects can include mucositis, esophagitis, peripheral neuropathy, or bone marrow suppression.
Using KNOWoncology.org
To review the evidence for natural therapeutic options in KNOWoncology.org, we start our search by limiting the tumor type to lung cancer. This results in 116 studies on natural interventions and lung cancer. One can quickly at a glance scan the titles and year of publication listed for statistically significant positive studies (highlighted in green), mixed results studies (highlighted in yellow), and negative studies (highlighted in red). The higher level-of-evidence studies are found at the top and include systematic reviews, meta-analyses, and clinical trials. Studies with higher risk of bias and considered to be “lower” level evidence, ie, observational studies and case reports, are listed at the bottom. Clicking on any citation title will expand it to reveal the full, peer-reviewed summary chart with detailed information on cancer type, treatment type, natural therapy, dose, duration, adverse effects, and outcomes.
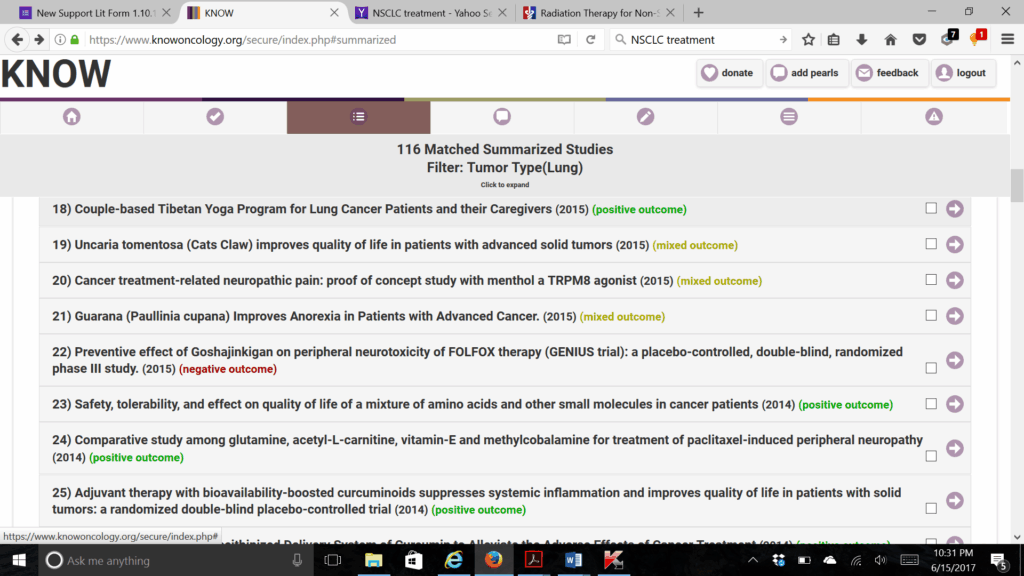
Figure 1. Sample List of Studies Matching Search Criteria
Improving Survival Time
Limiting the lung cancer studies above to “outcome type/survival,” results in 15 matched studies. This is very exciting! We see that a randomized controlled trial (RCT) reports improved 5-year survival in lung cancer patients by adding melatonin 20 mg4 to chemotherapy, also that an earlier controlled trial shows that melatonin 20 mg improved 1-year survival.5 We also see that, according to a meta-analysis of over 1500 patients with NSCLC, those who used Astragalus-based herbal combinations along with platinum-based chemotherapy had statistically significant improvement in overall survival compared with those who used platinum-based chemotherapy alone.6 One, 2, and 3-year survival rates were all better in the Astragalus-based groups, along with better quality of life. There was also less nausea/vomiting, fatigue, lack of appetite, and low blood counts in the people that used Astragalus-based combinations compared to platinum-based therapy alone.6 We can also learn from closer inspection that the greatest benefit was found in Astragalus-based herbal formulas that were based on syndrome differentiation (the Traditional Chinese Medicine form of diagnosis) versus Astragalus-based injection or Astragalus-based, oral patent medicines. The findings in this review support “individualized treatment” – one of the founding principles of naturopathic medicine – as a method that can actually confer a survival benefit in lung cancer!
Side Effect Management
Esophagitis
Esophagitis is a common and distressing side effect of chemo-radiation (radiation concurrent with chemotherapy) for lung cancer. Esophagitis can make swallowing so painful that patients stop eating, lose weight, get surgically-placed feeding tubes, start liquid diets, get depressed, and spiral downward in terms of vitality and hope. An important clinical pearl for chemo-radiation is that it is essential to prioritize supplements and be prepared to switch to powdered or liquid forms if needed.
By searching KNOW for side effect and esophagitis, we discover 3 small but pertinent studies. One of these is an observational study on oral glutamine. It reports that people with stage III NSCLC who took oral glutamine (10 g TID) during their chemo-radiotherapy had less weight loss and less severe inflammation of the esophagus compared to those who did not take glutamine.7 Glutamine supplementation also improved disease-free survival and showed a trend toward better overall survival (p=.05). In another study, lung cancer patients with grade II esophagitis from chemo-radiation were given oral epigallocatechin-3-gallate (EGCG) solution TID, 1 of 6 different concentration levels, 2 hours before radiation.8 Dramatic regression of esophagitis was observed in 22 of 24 patients. Grade II esophagitis persisted in 2 of 24 patients at the end of radiotherapy. Pain scores decreased further during every week of treatment (normally pain would increase over time). The most effective concentration was 440 µmol/L and was recommended for subsequent study. Adverse effects of EGCG included grade II heartburn (n=1) and stomach discomfort (n=1), and most patients complained about its nauseating taste.8 The last study that came from our esophagitis search was an elemental diet trial that included glutamine – a study that had mixed outcomes.9
Peripheral Neuropathy
Peripheral neuropathy is often a dose-limiting side effect of chemotherapy. When searching KNOW, we could either select for side effect and peripheral neuropathy or, alternatively, we could just use the open text box to search the agent being used, eg, carboplatin. With these searches, we have learned from KNOW that options to prevent neuropathy include fish oil, glutamine, and vitamin E, and that acupuncture shows effectiveness for established neuropathy. In a systematic review of 10 randomized clinical trials, omega-3 supplements during chemotherapy and/or radiation therapy helped patients maintain weight, reduced peripheral neuropathy, reduced inflammation (as reflected by C-reactive protein), and improved quality of life.10 It did not, however, impact survival times. In a systematic review of 3 clinical trials, oral glutamine prevented chemotherapy-induced neuropathy in cancer patients and reduced interference with activities of daily living.11 While glutamine supplementation in cancer patients is controversial, we use it routinely during chemotherapy because glutamine becomes a conditionally essential amino acid when there is rapid cell turnover. There are 4 studies on vitamin E (300 mg BID alongside platinum or taxol chemotherapy) in which vitamin E significantly reduced peripheral neuropathy.12-15 Lastly, in a systematic review of 7 clinical trials, acupuncture proved beneficial for existing chemotherapy-induced peripheral neuropathy.16
Bone Marrow Suppression
Low white blood cell (WBC) counts delay chemotherapy and leave patients vulnerable to infections. Astragalus-based formulas, as discussed earlier, constitute well-researched supportive care for maintaining immunity and improving survivorship. Other strategies revealed through KNOW, with varying levels of evidence, include wheatgrass juice (60 mL),17 melatonin (20 mg),18 Coriolus versicolor,19 oral shitake hot-water extract,20 and shenqi fuzheng injections.21 Empirically, Chinese herbal combinations including Spatholobus / Milletia (aka Ji Xue Teng) appear to be particularly effective at maintaining WBC counts during chemotherapy.
Quality of Life
Subcutaneous Viscum album (mistletoe) is an option for advanced cancer patients that is used worldwide. In KNOW, we can see a large RCT from 2004, reporting that a proprietary mistletoe extract injection alongside chemotherapy improved quality of life and decreased side effects such as pain, nausea, fatigue, anorexia, and insomnia in cancer patients compared to controls.22 We can also see a smaller RCT from 2013 that had mixed results using another common mistletoe preparation.23 In this study, there were no differences in median overall survival or quality of life in the patients taking mistletoe; however, they did have fewer chemotherapy dose reductions, fewer grade 3-4 toxicities, and fewer hospitalizations.23
Weighing the Evidence for Therapies & Interactions
Some of the most difficult decisions in naturopathic oncology concern herb-drug or nutrient-drug interactions, as well as understanding when the benefits of using specific therapies might outweigh the risks. Also, newer medications like targeted therapies and immunotherapies are not well understood, so little is known about how natural agents interact with them and even how these drugs interact with each other.
Herb-Drug-Supplement Interactions
Physicochemical interactions are fairly straightforward and are responsible for physical interactions that occur in the gastrointestinal tract resulting in changes in a drug’s bioavailability. This type of reaction can be avoided by dosing a fiber supplement at the opposite time of day as erlotinib.
Pharmacokinetic herb-drug interactions are more complex because they can lead to increased drug toxicity, on one hand, or reduced efficacy, on the other. This type of interaction arises from concurrent use that affects drug metabolism, distribution, and elimination. Pharmacokinetic interactions occur predominantly via changes in actions of the cytochrome enzymes mostly present in the liver, but also present in other tissues, including the brush border of the intestines. With intravenous chemotherapy, these types of interactions are best avoided by timing the application of herbs in windows separated by at least 3 half-lives away from the drug in question.24 Oral medications present more of a problem. Compared to intravenous chemotherapy, oral chemotherapy agents go through first-pass metabolism in the liver, persist in the bloodstream, and are more likely to have drug interactions. In this particular case, erlotinib is a major CYP3A4 substrate and is metabolized to a lesser extent by CYP1A2 and CYP1A1.25 It also inhibits CYP2C9.26 Since erlotinib does not act as an oxidative cytotoxic agent (the way most chemotherapeutic drugs work), from a practical standpoint it is at least unlikely that there are pharmacodynamic interactions to be concerned about when including antioxidants or anti-inflammatory agents.
Musings on Curcumin
The use of curcumin alongside erlotinib is controversial. In 2014, we believed curcumin should not be used with erlotinib because curcumin is a potential CYP3A4 inducer that could decrease circulating drug levels. This belief was based on a concerning pre-clinical study in rats.27 However, opinions changed in 2016 based on 3 newer studies. The first study was a randomized, placebo-controlled, crossover trial in which piperine-enhanced curcuminoids produced no meaningful changes in plasma Cmax, AUC, clearance, elimination half-life, or metabolite levels of the drug-probes midazolam, flurbiprofen, or acetaminophen (α=0.05, paired t-tests), all of which are metabolized by CYP3A, CYP2C9 and acetaminophen conjugation enzymes.28 As well, both an in-vitro study29 and mouse study30 were published showing that curcumin enhanced erlotinib’s anti-tumor activity.
Summary
When working with cancer patients, naturopathic doctors are known to combine current best evidence, clinical experience, and patient preferences. In this way, standard-of-care treatment for cancer is enhanced and patients can be treated individually. Utilizing the research tool KNOWoncology.org, relevant research can be quickly accessed and used to help formulate patient treatment protocols.
In Part 2 of this article, we will present 2 cases of patients with NSCLC that were treated in an integrative manner. The cases will serve to demonstrate the application of KNOW in clinical practice.
References:
- Non-Small Cell Lung Cancer Survival Rates, by Stage. Last revised May 16, 2016. American Cancer Society Web site. https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed June 20, 2017.
- Shaw AT, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naïve advanced ALK-positive non-small cell lung cancer (NSCLC): Primary results of the global phase III ALEX study. [abstract LBA9008, presented at 2017 ASCO Annual Meeting]. J Clin Oncol. 2017;35(suppl; abstr LBA9008). Available at: http://abstracts.asco.org/199/AbstView_199_185951.html. Accessed June 15, 2017.
- Treatment Choices for Non-Small Cell Lung Cancer, by Stage. Last revised June 23, 2017. American Cancer Society. https://www.cancer.org/cancer/non-small-cell-lung-cancer/treating/by-stage.html. Accessed June 20, 2017.
- Lissoni P, Chilelli M, Villa S, et al. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003;35(1):12-15.
- Lissoni P, Barni S, Mandalà M, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 1999;35(12):1688-1692.
- Wang SF, Wang Q, Jiao LJ, et al. Astragalus-containing Traditional Chinese Medicine, with and without prescription based on syndrome differentiation, combined with chemotherapy for advanced non-small-cell lung cancer: a systemic review and meta-analysis. Curr Oncol. 2016;23(3):e188-195.
- Gul K, Mehmet K, Meryem A. The effects of oral glutamine on clinical and survival outcomes of non-small cell lung cancer patients treated with chemoradiotherapy. Clin Nutr. 2017;36(4):1022-1028.
- Zhao H, Zhu W, Xie P, et al. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother Oncol. 2014;110(1):132-136.
- Tanaka Y, Takahashi T, Yamaguchi K, et al. Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: a feasibility study. Support Care Cancer. 2016;24(2):933-941.
- de Aguiar Pastore Silva J, Emilia de Souza Fabre M, Waitzberg DL. Omega-3 supplements for patients in chemotherapy and/or radiotherapy: A systematic review. Clin Nutr. 2015;34(3):359-366.
- Amara S. Oral glutamine for the prevention of chemotherapy-induced peripheral neuropathy. Ann Pharmacother. 2008;42(10):1481-1485.
- Argyriou AA, Chroni E, Koutras A, et al. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: final results. Support Care Cancer. 2006;14(11):1134-1140.
- Pace A, Savarese A, Picardo M, et al. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin J Clin Oncol. 2003;21(5):927-931.
- Argyriou AA, Chroni E, Koutras A, et al. Preventing paclitaxel-induced peripheral neuropathy: a phase II trial of vitamin E supplementation. J Pain Symptom Manage. 2006;32(3):237-244.
- Argyriou AA, Chroni E, Koutras A, et al. Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology. 2005;64(1):26-31.
- Franconi G, Manni L, Schroder S, et al. A systematic review of experimental and clinical acupuncture in chemotherapy-induced peripheral neuropathy. Evid Based Complement Alternat Med. 2013;2013:516916.
- Bar-Sela G, Tsalic M, Fried G, Goldberg H. Wheat grass juice may improve hematological toxicity related to chemotherapy in breast cancer patients: a pilot study. Nutr Cancer. 2007;58(1):43-48
- Lissoni P, Tancini G, Barni S, et al. Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Support Care Cancer. 1997;5(2):126-129.
- Fritz H, Kennedy DA, Ishii M, et al. Polysaccharide K and Coriolus versicolor extracts for lung cancer: a systematic review. Integr Cancer Ther. 2015;14(3):201-211.
- Nagashima Y, Maeda N, Yamamoto S, et al. Evaluation of host quality of life and immune function in breast cancer patients treated with combination of adjuvant chemotherapy and oral administration of Lentinula edodes mycelia extract. Onco Targets Ther. 2013;6:853-859.
- Li Y, Han N, Chen Z, Li X. Systematic review and meta-analysis of shenqi fuzheng and chemotherapy combination in the treatment of breast cancer. Bangladesh Journal of Pharmacology. 2016;11(4):793-801.
- Piao BK, Wang YX, Xie GR, et al. Impact of complementary mistletoe extract treatment on quality of life in breast, ovarian and non-small cell lung cancer patients. A prospective randomized controlled clinical trial. Anticancer Res. 2004;24(1):303-309.
- Bar-Sela G, Wollner M, Hammer L, et al. Mistletoe as complementary treatment in patients with advanced non-small-cell lung cancer treated with carboplatin-based combinations: a randomized phase II study. Eur J Cancer. 2013;49(5):1058-1064.
- Seely D, Stempak D, Baruchel S. A strategy for controlling potential interactions between natural health products and chemotherapy: a review in pediatric oncology. J Pediatr Hematol Oncol. 2007;29(1):32-47.
- Sasu-Tenkoramaa Fudin J. Drug Interactions in Cancer Patients Requiring Concomitant Chemotherapy and Analgesics. Practical Pain Management. May 2013. Available at: https://www.practicalpainmanagement.com/pain/cancer/chemotherapy-neuropathy/drug-interactions-cancer-patients-requiring-concomitant?page=0,4. Accessed June 20, 2016.
- Grenader T, Gipps M, Shavit L, Gabizon A. Significant drug interaction: phenytoin toxicity due to erlotinib. Lung Cancer. 2007;57(3):404-406.
- Hsieh YW, Huang CY, Yang SY, et al. Oral intake of curcumin markedly activated CYP 3A4: in vivo and ex-vivo studies. Sci Rep. 2014;4:6587.
- Volak LP, Hanley MJ, Masse G, et al. Effect of an herbal extract containing curcumin and piperine on midazolam, flurbiprofen, and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2013;75(2):450-462.
- Li S, Liu Z, Zhu F, et al. Curcumin lowers erlotinib resistance in non-small cell lung carcinoma cells with mutated EGF receptor. Oncol Res. 2013;21(3):137-144.
- Yamauchi Y, Izumi Y, Yamamoto J, Normori H. Coadministration of erlotinib and curcumin augmentatively reduces cell viability in lung cancer cells. Phytother Res. 2014;28(5):728-735.
Image Copyright: <a href=’https://www.123rf.com/profile_ciphotos’>ciphotos / 123RF Stock Photo</a>
 Jen Green, ND, FABNO, graduated from CCNM in 2000. In 2008, she founded the Naturopathic Department at Beaumont Hospitals, and was the department head for 5 years. Dr Green has served on various naturopathic boards, including the OAND, CAND, MANP, and the Oncology Association of Naturopathic Physicians. She is currently one of the research directors of KNOW, the Knowledge in Naturopathic Oncology Website. Jen is currently in private practice at Emcura Integrative in Bloomfield Hills, Michigan.
Jen Green, ND, FABNO, graduated from CCNM in 2000. In 2008, she founded the Naturopathic Department at Beaumont Hospitals, and was the department head for 5 years. Dr Green has served on various naturopathic boards, including the OAND, CAND, MANP, and the Oncology Association of Naturopathic Physicians. She is currently one of the research directors of KNOW, the Knowledge in Naturopathic Oncology Website. Jen is currently in private practice at Emcura Integrative in Bloomfield Hills, Michigan.
 Dugald Seely, ND, MSc, FABNO, is a naturopathic doctor, researcher, and administrator who has actively contributed since graduating from CCNM in 2003. Dr Seely serves as the executive director of Research & Clinical Epidemiology at CCNM. He is also the founder and executive director of the Ottawa Integrative Cancer Centre, a Fellow of the American Board of Naturopathic Oncology, and an executive board member for the Oncology Association of Naturopathic Physicians. Dr Seely is a section editor for Integrative Oncology in the journal Current Oncology, has published over 60 peer-reviewed papers, and frequently presents at medical conferences around the world.
Dugald Seely, ND, MSc, FABNO, is a naturopathic doctor, researcher, and administrator who has actively contributed since graduating from CCNM in 2003. Dr Seely serves as the executive director of Research & Clinical Epidemiology at CCNM. He is also the founder and executive director of the Ottawa Integrative Cancer Centre, a Fellow of the American Board of Naturopathic Oncology, and an executive board member for the Oncology Association of Naturopathic Physicians. Dr Seely is a section editor for Integrative Oncology in the journal Current Oncology, has published over 60 peer-reviewed papers, and frequently presents at medical conferences around the world.
 Heather Wright, ND, FABNO, is a naturopathic doctor with 12 years of experience working within hospital-based integrative oncology teams. Dr Wright has specialized in the integrative treatment of pancreatic cancer and has co-authored clinical trials to assess the benefit of natural approaches in this population. She is also a regular contributor to the Natural Medicine Journal and as a speaker provides continuing education to colleagues. She is President of the Oncology Association of Naturopathic Physicians (OncANP.org) and serves as Research Director for KNOWoncology.org, a searchable database of human clinical trials in integrative oncology. Dr Wright currently works at Goodapplewellness.com.
Heather Wright, ND, FABNO, is a naturopathic doctor with 12 years of experience working within hospital-based integrative oncology teams. Dr Wright has specialized in the integrative treatment of pancreatic cancer and has co-authored clinical trials to assess the benefit of natural approaches in this population. She is also a regular contributor to the Natural Medicine Journal and as a speaker provides continuing education to colleagues. She is President of the Oncology Association of Naturopathic Physicians (OncANP.org) and serves as Research Director for KNOWoncology.org, a searchable database of human clinical trials in integrative oncology. Dr Wright currently works at Goodapplewellness.com.