Rebeccah Shalev, ND
Honorable Mention Research Review
Conventional treatments for PCOS generally focus on controlling symptoms related to the anovulation, insulin resistance, and/or hyperandrogenism common to the syndrome. Different medications – none without side effects – are utilized for each of the aforementioned 3 types of symptoms.1 Elevated androgen levels typically manifest in women as hirsutism, female-pattern baldness, and acne. These symptoms can be quite distressing to women with PCOS, hence the need for treatment options for female hyperandrogenism that are both safe and effective. Mentha spicata, or common spearmint, holds promise as just such a treatment. Polycystic Ovarian Syndrome (PCOS) is one of the most common female endocrine disorders,1 occurring in up to 10% of women attending gynecology clinics.2 It is a syndrome of ovarian dysfunction that typically presents in women of childbearing age, with signs and symptoms of hyperandrogenism, polycystic ovaries, menstrual irregularities, obesity, and insulin resistance. It is also frequently associated with elevated serum luteinizing hormone (LH) levels and increased risk of type 2 diabetes and cardiovascular events.3 The 2003 Rotterdam criteria for diagnosing PCOS require the presence of 2 of the 3 following criteria: androgen excess, ovulatory dysfunction, and/or polycystic ovaries, in exclusion of other causative diagnoses.4
This paper reviews a controlled study that compared spearmint to placebo in the reduction of symptoms of androgen excess in women with a clinical diagnosis of PCOS. Outcome measures were serum androgen levels and subjective and objective assessments of hirsutism severity.5
Literature Review
The author conducted an extensive literature review of controlled trials of phytotherapeutic treatments for PCOS and its common symptoms. The search was limited to articles published from 2003 to 2013 in journals that were either open-access or available through Bastyr University subscription. Key search terms included PCOS, polycystic ovarian syndrome, polycystic ovary syndrome, androgen excess, androgen, hyperandrogenism, hyperandrogenemia, hirsutism, acne, alopecia, 5-alpha reductase, phytotherapy, phytotherapeutic, herb, herbal, botanical, complementary and alternative medicine, CAM, and the common and Latin names of herbs that have traditionally been used for treatment of PCOS and/or hyperandrogenism (eg, saw palmetto, soy, green tea, licorice, black cohosh, nettles, sarsaparilla, bloodroot, turmeric, red reishi, Chinese peony, chaste tree, and spearmint).
After excluding articles not relevant to the patient population in question (ie, articles focusing on male-pattern baldness and/or benign prostatic hypertrophy, or the PCOS symptoms of obesity and/or insulin resistance), 3 articles remained that examined treatments for androgen excess in women with PCOS. One5 studied the effects of an herb (spearmint), another6 studied the effects of a food (soy), and a third7 studied the effects of an herbal mixture (Tian Gui) on androgen excess in women with PCOS. While the reported results of the Tian Gui formula were impressive, it was unclear whether they were attributable to 1, to several, or to a synergistic combination of all of the 11 ingredients. It was also unlikely that the Chinese ingredients in this formula would be as familiar and as easy to obtain by patients in this country, as would spearmint and soy. Only the spearmint study measured the effects of one easily obtainable natural product on not only androgenic hormone levels, but also one of the most common clinically observable effects of these increased androgen levels: the severity of hirsutism in the study participants.
The Spearmint Trial
The spearmint trial conducted by Grant (2010)5 administered either spearmint tea or a chamomile tea placebo BID to 42 women with PCOS and hirsutism over a period of 1 month. Participants were 19-42 years old, with a mean age of 25.5 years, and were recruited from 2 district general hospitals in the United Kingdom. All had visible hirsutism and a diagnosis of PCOS, confirmed by the Rotterdam criteria of 2003.3,4 No exclusion criteria were listed. Participants were randomly assigned via computer to either the intervention group or to a control group (both groups began the study with 21 participants; 1 dropped out of the control group due to dislike of chamomile tea). Groups were apparently not matched for secondary characteristics, such as age, body mass index, level of hirsutism or serum androgens, or length of PCOS diagnosis.
Participants consumed 2 cups of tea per day, made from tea bags provided with a pre-measured, standardized content of dried herb. Participants were instructed to prepare the tea to a standard strength at home over the course of 30 days, beginning on the day after the cessation of each participant’s menstrual period. Intervention group members received spearmint tea, and control group members received chamomile, which is not known to affect hormonal levels. While researchers were blinded as to which tea was given to each patient, patients were likely aware of which tea they had been given, based upon the flavor of the tea. However, it is unknown whether patients were aware of which herb was considered the intervention and which the placebo. There was no mention of an intention-to-treat analysis being performed. No adverse effects were reported.
Outcomes measured in the study included serum levels of free testosterone (FT), total testosterone (TT), dehydroepiandrosterone sulfate (DHEA-S), luteinizing hormone (LH), and follicle-stimulating hormone (FSH), measured on the first, last, and 15th day of each participant’s 30-day study period. In addition, on the first and last days of the study, each participant completed a Dermatology Quality of Life Index questionnaire (DQLI),8 to assess her own current level of hirsutism. Two separate clinicians also objectively evaluated each participant on the first and last day of the study. Level of hirsutism was assessed by the Ferriman-Gallwey index.9 Inter-observer correlation was reported to be “good [and] reliable,” although reliability statistics were not provided.
Results
Participants were evaluated within the groups to which they were assigned, and scores were reported as averages for each group. While the levels of LH and FSH increased by a statistically significant amount over the course of the study in the spearmint group (p<0.05), the levels of FT and TT appeared to be the most dramatically affected by the intervention (p<0.05). In the spearmint group, the FT average on day 0 was 5.12 ± 2.14 pg/mL, and on day 30 it was 3.64 ± 2.67 pg/mL, showing a net reduction of 28.9% over the course of the study. In the control group, FT on day 0 was 4.98 ± 2.84 pg/mL, and on day 30 was 4.49 ± 1.67 pg/mL, showing a net reduction of only 9.8%. The TT average for the spearmint group measured 0.81 ± 0.39 ng/mL on day 0 and 0.62 ± 0.34 ng/mL on day 30, showing a net reduction of 23.5%. In the control group, the TT average measured 0.87 ± 0.40 ng/mL on day 0 and 0.80 ± 0.14 ng/mL on day 30, for a net reduction of 8.1% over the course of the study.
Clinical and self-assessments of hirsutism were also performed on days 0 and 30 of the trial. Patients’ self-assessments using the DQLI showed a significant reduction in the spearmint group (p<0.05). Reduction in clinically observable levels of hirsutism using the objective Ferriman-Gallwey index approached but did not reach significance (p=0.12). Not enough data was provided to calculate the Experimental Event Rate, Relative Risk, Relative Risk Reduction, Absolute Risk Reduction, or Number Needed to Treat.
Discussion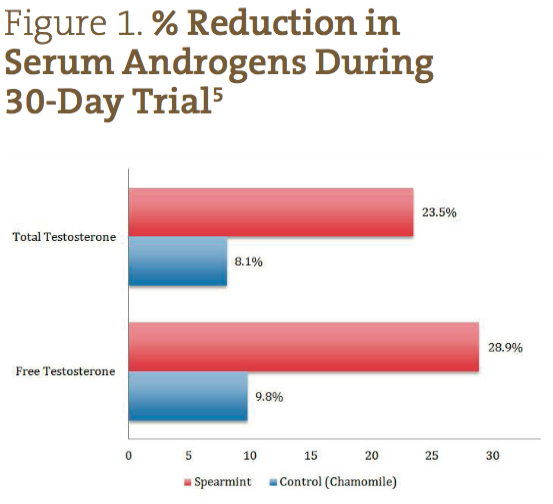
The primary endpoint of the spearmint study was to demonstrate, via consumption of spearmint tea, a reduction in hirsutism in women with PCOS, as quantified by a modified DQLI and Ferriman-Gallwey score. This endpoint was partially reached, with a significant improvement in the DQLI but not with the Ferriman-Gallwey score. The study’s secondary endpoint was to demonstrate a significant reduction in serum androgen levels; this endpoint was reached, with a significant reduction in FT and TT levels.
The failure to attain a significantly reduced Ferriman-Gallwey score may be explained by the study’s duration being too short to observe such changes. Hair growth goes through cyclical changes, with phases of growth, rest, and release varying in length of time among different people and on different parts of the body. Based on results of drug studies on hirsutism, a longer trial of several months may be required to clinically confirm the effects of spearmint tea on hirsutism.10
In addition to the insufficient study duration, other limitations of the spearmint study include the method of administration of the spearmint and chamomile teas. Study participants prepared their own teas at home, and even though they had been given pre-measured tea bags and instructions for uniform preparation, the possibility remained for errors and/or variations in patient tea preparation affecting the strength of the teas. In addition, administering the herbs by way of a tea removed the ability to effectively blind the study participants as to their assigned spearmint/chamomile groups. A recommendation for future study would be to provide study participants with standardized encapsulations of dried herb or extract, rather than requiring them to brew their own teas twice a day.
Conclusions
Patients in this study were similar to PCOS patients seen in a typical gynecological practice in this country; thus, study results obtained can be expected to be applicable to clinical practice. Spearmint herbal tea was found to be a promising treatment option, reducing serum levels of free and total testosterone by a statistically significant amount in only 30 days. As the link between hirsutism and androgenic hormones is already well established,10,11 it can be extrapolated that this reduction in androgenic hormones would likely translate into a clinically observable reduction in hirsutism in women with PCOS, assuming the therapy is given enough time to act on the different phases of the hair cycle. A longer study is required to confirm this prediction.
Spearmint is considered by the US Food and Drug Administration to be “Generally Recognized as Safe,” both in its fresh herbal state and as an essential oil extract.12 The herb has a long history of safe use for culinary purposes, and is inexpensive and widely available. The spearmint plant is also easy to grow in many climates, and with its low requirement for root space and direct sunlight, it can thrive even in a window box or in a small pot on a porch or balcony, making home cultivation accessible to the vast majority of patients.
Given spearmint’s association with a significant decrease in serum androgen levels, as well as the lack of any adverse events reported in this study, the herb’s potential benefits to patients with androgen excess from PCOS easily outweigh any likely risk. At this time, clinicians need not hesitate in recommending spearmint tea to women with signs of androgen excess.
 Rebeccah Shalev, ND, wrote this article while still a student Bastyr University. She has since graduated and is now completing a residency at the Factoria Women and Family Clinic in Bellevue, WA. She has a special interest in the interplay between environmental toxicology, endocrinology, chronic infections, neurology, and brain disorders. As a certified Autonomic Response Testing practitioner, Dr Shalev enjoys getting to the bottom of complex and/or elusive diagnostic challenges, such as those seen with syndromes like fibromyalgia, multiple chemical sensitivities, and chronic Lyme disease. She is also passionate about organic gardening, and spearmint is one of her favorite herbs.
Rebeccah Shalev, ND, wrote this article while still a student Bastyr University. She has since graduated and is now completing a residency at the Factoria Women and Family Clinic in Bellevue, WA. She has a special interest in the interplay between environmental toxicology, endocrinology, chronic infections, neurology, and brain disorders. As a certified Autonomic Response Testing practitioner, Dr Shalev enjoys getting to the bottom of complex and/or elusive diagnostic challenges, such as those seen with syndromes like fibromyalgia, multiple chemical sensitivities, and chronic Lyme disease. She is also passionate about organic gardening, and spearmint is one of her favorite herbs.
References
Ajossa S, Guerriero S, Paoletti AM, et al. The treatment of polycystic ovary syndrome. Minerva Ginecol. 2004;56(1):15-26.
Cahill D. PCOS. BMJ Clin Evid. 2009;2009. pii: 1408.
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592.
Grant P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother Res. 2010;24(2):186-188.
Khani B, Mehrabian F, Khalesi E, Eshraghi A. Effect of soy phytoestrogen on metabolic and hormonal disturbance of women with polycystic ovary syndrome. J Res Med Sci. 2011;16(3):297-302.
Kuek S, Wang WJ, Gui SQ. Efficacy of Chinese patent medicine Tian Gui Capsule in patients with polycystic ovary syndrome: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2011;9(9):965-972.
Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216.
Ferriman,D. Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440-1447.
Lai JJ, Chang P, Lai KP, et al. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res. 2012;304(7):499-510.
Ruutiainen K, Erkkola R, Kaihola HL, et al. The grade of hirsutism correlated to serum androgen levels and hormonal indices. Acta Obstet Gynecol Scand. 1985;64(8):629-633.
U.S. Food and Drug Administration. Code of Federal Regulations Title 21. Part 182 – Substances Generally Recognized As Safe. Last updated August 21, 2015. Available at: http://tinyurl.com/nhng3f8. Accessed December 15, 2013.