Angela Cortal, ND
Osteoarthritis (OA) is a chronic, progressive condition of worsening joint destruction that affects most of us in the United States. Even if you do not personally experience OA, you likely have a friend or family member who does. In the United States, alone, the incidence of OA has risen from an estimated 21 million in 1990 to almost 27 million adults today,1 affecting nearly 38% of those ages 45 years and older, and almost 48% of those ages 65 years and older.2Although research continues to develop in this field, conventional treatment options have not stemmed the worsening prevalence (ie, there is no disease-modifying drug or other conventional intervention that accomplishes this3).
Idiopathic OA of the Knee
This article is focused on idiopathic OA of the knee (the most commonly affected joint), as opposed to OA that develops secondary to other pathologic processes such as trauma, congenital etiology, or crystalline deposition. Often called degenerative joint disease (DJD), OA is the more accurate term for the condition. Although there is degenerative joint pathology at hand, the inflammatory pathophysiology is now accepted to be far more complex than the simple “wear and tear” that was previously described to be the primary etiology.
After a patient has been ruled out for secondary pathology, he or she may be screened for rheumatologic and inflammatory markers (such as sedimentation rate and rheumatoid factor). Patients are diagnosed with knee OA when they meet at least 3 of the 6 diagnostic criteria developed in 1981 by the American College of Rheumatology; this diagnostic approach has a sensitivity of 95% and a specificity of 69% (Table 1).4 Additional radiographic findings, if performed, include loss of articular cartilage and bony growths.
Table 1: Diagnostic Criteria for Knee OA4
| Greater than 50 years of age |
| Morning stiffness for less than 30 minutes |
| Crepitus on active motion of the knee |
| Bony tenderness |
| Bony enlargement |
| No palpable warmth |
Naturopathic medicine is perfectly positioned to provide relief for the symptoms of OA, address the underlying triggers and modifiable risk factors, as well as modulate the pathophysiology of this chronic degenerative and inflammatory condition. One of the primary etiologies of OA is the reduction of chondrocyte proliferation, with a compensatory proliferation of osteocytes within an environment of increased inflammatory cytokines.5 Ie, the cartilage diminishes as a response to the local inflammatory state and is replaced with bone cell growth. This is often explained to patients after an X-ray as a “bone on bone” joint.
Risk Factors & Conventional Treatments
Established modifiable risk factors include increased body mass index (having a BMI categorized as obese or greater)6; history of trauma or injury7; physical labor duties (particularly those involving repetitive tasks or which cause mechanical stress to joints, muscles, and body mechanics)8; hormone deficiency (particularly in women )9; nutritional deficiency; and abnormal bone density.10
Non-modifiable risk factors for the physician to keep in mind include gender (women have a higher incidence than men), age, and genetic predispositions. Thinking comprehensively, a naturopathic doctor may also add to the list any systemic condition which triggers or worsens inflammatory processes, as elevated and prolonged inflammatory cascades are at the heart of OA’s pathophysiology.11
Treatment goals in both conventional and naturopathic medicine often emphasize reductions in swelling, pain, and immobility. First-line therapy often involves exercise counseling, physical therapy referral, and addressing identified modifiable risk factors as described above. Initial pharmacotherapy recommendations often include acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs) for the purposes of analgesia and inflammation reduction.12 Failing these treatment options, intra-articular glucocorticoid injections are often the next step.13
Though both NSAIDs and intra-articular glucocorticoid injections have shown benefit in reduction of pain and swelling of the affected joint, there are considerations to be made as to NSAIDs’ long-term use and repeated steroid injection outcomes. The former has been shown to lead to increased incidence of many troubling conditions, such as gastritis, gastrointestinal bleeding,14 heart disease, and stroke.15 Due to multiple inhibitory effects of NSAIDs on cartilage repair and regeneration, healthcare practitioners have been warned that NSAIDs accelerate articular cartilage16 – the very pathophysiology that they are recommended to treat.
In addition, the physician who is keeping long-term health in mind for their patients should be aware that intra-articular steroid injections show indeterminate benefit at 12-month follow-ups,17 and in some studies result in compromised strength and integrity of joint tissue,18 as well as accelerated joint-space narrowing, as evidenced radiographically.19 For this reason the American Academy of Orthopedic Surgeons does not recommend this treatment for knee OA.20
That leaves patients who fail to respond to physical therapy and exercise rehabilitation with treatment options that present many potential side effects from long-term use, and/or potentially provide minimal or no benefit over time. A patient’s next steps in conventional care are often arthroscopic surgery (which may be no more effective than placebo21) or eventual total joint replacement, which many patients prefer to avoid if possible. I would like to present some naturopathic therapeutic options to consider, ones I utilized with a patient in the following case study.
Case Study
E.C. is a 53-year-old female whom I met as a referral from a local acupuncturist. The patient was already physically active and paid close attention to stress management and work/life balance, regularly incorporating yoga, meditation, and acupuncture into her health regimen.
She came to me primarily because of joint pain, which she had experienced for decades. Overall, she had improved with the above measures, though the pain in her knees continued to prevent her from achieving certain yoga poses (eg, those requiring 1-legged balance and stable knee positioning) as well a goal of hers to be even more physically active – something which we always love to support.
E.C. had been thoroughly worked up in the past, was ruled out for rheumatoid arthritis as well as other infectious, autoimmune, and inflammatory etiologies of her joint pain. Her only past remarkable lab was elevated C-reactive protein. As OA is a diagnosis of exclusion, she had also had an X-ray which showed mild osteoarthritic changes in her knees. This resulted in a diagnosis of OA prior to our work together.
She and I agreed to first work on systemically and holistically addressing inflammation, and see to what effect this would reduce her joint pain and improve her mobility and physical capacity.
Treatment
We began with supplementation of curcumin (bioavailable form, 500 mg BID22) and essential fatty acids (1.5 g fish oil23), along with a discussion of potential dietary triggers and elimination/reintroduction trials. The bioavailable curcumin was added in order to downregulate inflammatory enzymes cyclooxygenase (COX)-1 and COX-2, as well as inflammatory cytokines, such as nuclear factor-kappaB (NF-ĸB), which lead to downstream dampened expression of matrix metalloproteinase, a very important element in connective tissue degradation.24 The essential fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) both increase the anti-inflammatory prostaglandin E3 and reduce expression of COX-1 and COX-2; DHA also promotes a reduction in the release of reactive oxygen species into the body.25
Follow-up Visits
One-month follow-up: The severity of E.C.’s arthralgias was improved, though the dietary change recommendations were met with resistance. Her significant other was unsupportive of dietary changes in the home (which is often the case), so we continued on with our original treatment plan, incorporating those changes she could make. We incidentally also found that she was mildly hypothyroid, which can be its own independent risk factor for the development of OA,26 as skeletal tissue is highly T3-sensitive.
Three-month follow-up & treatment: By addressing both systemic inflammation and her newly-found hypothyroidism, we saw substantial improvement at 3 months in her daily joint pains. However, they were still persistent enough to prevent her from riding a bike and climbing stairs without pain – 2 of her primary mobility-based goals.
E.C.’s physical exam continued to reveal multiple areas of ligament, tendon, and joint line tenderness around her left knee, as well as mild instability of both knees (as found on varus and valgus stress testing). It was at this time that we discussed prolotherapy as a potential beneficial addition to her treatment plan. There is strong positive evidence supporting the use of prolotherapy in knee OA,27 with reduction in pain and swelling, and improved mobility seen repeatedly in research on 1-year follow-ups.28-30
Prolotherapy, along with platelet-rich plasma injections, falls within the category of Regenerative Injection Therapies, which are injections performed to joints, ligaments, tendons, and other areas of connective tissue (Figure 1). The intent is for the injected solution to prompt a local healing response,31 with a corresponding infiltration of one’s own stem cells, recruited to stimulate a regrowth of connective tissue that may have become injured or degenerative due to past trauma or chronic degenerative changes.32 It is via these mechanisms that the goals of pain reduction, swelling reduction, and improvements in strength and integrity of joints and other connective tissue are sought.
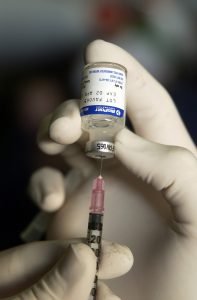
Two months later: In a 2-month follow-up visit after 1 session of 12.5% dextrose prolotherapy (performed to both areas of identified joint, ligament, and tendon instability, pain and pathology), E.C. exuberantly explained how she had just returned from a vacation (where she bike-commuted everywhere) and could finally perform the tree pose in her yoga class. The tree pose is a 1-legged balance pose, where the elevated leg is bent and contacting the standing supportive leg (Figure 2). Her prior knee instability and weakness had prevented her from performing this pose for years. Both she and I were very pleased with the progress she made after just 1 session.

Six-month follow-up: E.C. still had retained approximately 90% of the gains she made post-prolotherapy. At that point we discussed a potential second prolotherapy session, to be scheduled should she experience any further return of pain or instability.
This case study has been included specifically because of how clearly it illustrates the naturopathic approach to addressing one’s health.33 E.C. already had many foundations of health in place (which we continued to refine and build upon), so we began to specifically incorporate nutrients and a dietary plan to promote more anti-inflammatory activity; after that point we introduced Regenerative Injection Therapies. For E.C. this was a smart approach, so that we could see to what extent each element was improving her symptoms (with 1 of our primary goals being to affect underlying pathophysiology).
At this point we are both satisfied with the great gains she has seen with the integrative and holistic approach I was able to offer her. It is extremely fulfilling, as we all know, to help support someone on their way to improved quality of life. I look forward to hearing about what is next for her, now that she can be so much more active in her life.
References:
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
- Centers for Disease Control and Prevention. Osteoarthritis (OA). Last updated October 28, 2015. CDC Web site. http://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed April 26, 2016.
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. 2011;377(9783):2115-2126.
- Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039-1049.
- Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5(3):351-361.
- Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41(8):1343-1355.
- Felson DT. Risk factors for osteoarthritis: understanding joint vulnerability. Clin Orthoped Relat Res. 2004;(427 Suppl):S16-S21.
- Rossignol M, Leclerc A, Allaert FA, et al. Primary osteoarthritis of hip, knee and hand in relation to occupational exposure. Occup Environ Med. 2005;62(11):772-777.
- Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43(5):995-1000.
- Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18(1):24-33.
- Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11(1):35-44.
- [No authors listed]. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905-1915.
- Zhang W, Doherty M, Leeb BF, et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66(3):377-388.
- Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.
- Ghosh R, Hwang SM, Cui Z, et al. Different effects of the nonsteroidal anti-inflammatory drugs meclofenamate sodium and naproxen sodium on proteasome activity in cardiac cells. J Mol Cell Cardiol. 2016;94:131-144.
- Hauser RA. The Acceleration of Articular Cartilage Degeneration in Osteoarthritis by Nonsteroidal Anti-inflammatory Drugs. J of Prolotherapy. 2010;2(1):305-322.
- Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005328.
- Wernecke C, Barun HJ, Dragoo JL. The Effect of Intra-articular Corticosteroids on Articular Cartilage: A Systematic Review. Orthop J Sports Med. 2015;3(5):23-25.
- Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
- Jevsevar DS, Brown GA, Jones DL, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013;95(20):1885-1886.
- Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359(11):1097-1107.
- Belcaro G, Cesarone MR, Dugall M, et al. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. 2010:15(4):337-344.
- Chen Y, Huang YC, Lu WW. Low-dose versus high-dose fish oil for pain reduction and function improvement in patients with knee osteoarthritis. Ann Rheum Dis. 2016;75(1):e7.
- Nakagawa Y, Mukai S, Yamada S, et al. Short-term effects of highly bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19(6):933-939.
- Curtis CL, Hughes CE, Flannery CR, et al. N-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275(2):721-724.
- Bassett JH, Williams GR. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr Rev. 2016;37(2):135-187.
- Rabago D, Kijowski R, Woods M, et al. Association between disease-specific quality-of-life and magnetic resonance imaging outcomes in a clinical trial of prolotherapy for knee osteoarthritis. Arch Phys Med Rehabil. 2013;94(11):2075-2082.
- Rabago D, Patterson JJ, Mundt M, et al. Dextrose Prolotherapy for Knee Osteoarthritis: A Randomized Controlled Trial. Ann Fam Med. 2013;11(3):229-237.
- Rabago D, Zgierska A, Fortney L, et al. Hypertonic Dextrose Injections (Prolotherapy) for Knee Osteoarthritis: Results of a Single-Arm Uncontrolled Study with 1-Year Follow-Up. J Altern Complement Med. 2012;18(4):408-414.
- Rabago D, Patterson JJ, Mundt M, et al. Dextrose and Morrhuate Sodium Injections (Prolotherapy) for Knee Osteoarthritis: A Prospective Open-Label Trial. J Alt Comp Med. 2014;20(5):383-391.
- Rabago D, Slattengren A, Zgierska A. Prolotherapy in Primary Care Practice. Primary Care. 2010;37(1):65-80.
- What Is Prolotherapy? J of Prolotherapy, A Publication of Regenerative Medicine Techniques. Available at: http://www.journalofprolotherapy.com/index.php/what-is-prolotherapy/. Accessed May 8, 2016.
- The American Association of Naturopathic Physicians. Definitions of Naturopathic Medicine. AANP Web site. http://www.naturopathic.org/content.asp?contentid=59. Accessed May 2, 2016.
 Angela Cortal, ND, is a naturopathic physician who focuses on Regenerative Injection Therapy (prolotherapy and platelet-rich plasma Injections) and integrates this into her naturopathic clinical practice. She loves working with patients at Heart Spring Health in Portland, OR, and observes phenomenal recoveries from past injuries, degenerative joint conditions, and chronic pain or instability. In addition, Dr Cortal loves to write and present nationally and internationally on topics of naturopathic medicine and Regenerative Injection Therapy. She can be reached at [email protected].
Angela Cortal, ND, is a naturopathic physician who focuses on Regenerative Injection Therapy (prolotherapy and platelet-rich plasma Injections) and integrates this into her naturopathic clinical practice. She loves working with patients at Heart Spring Health in Portland, OR, and observes phenomenal recoveries from past injuries, degenerative joint conditions, and chronic pain or instability. In addition, Dr Cortal loves to write and present nationally and internationally on topics of naturopathic medicine and Regenerative Injection Therapy. She can be reached at [email protected].