Optimizing Thyroid Function
Connie Sanchez, ND
The American Association of Clinical Endocrinologists (AACE) reports that up to 27 million Americans may have some type of thyroid disorder, and that more than half of those remain undiagnosed (2008). In his book, Thyroid Power, Dr. Richard L. Shames concurs and writes, “Although extremely common, low thyroid is an unsuspected illness. Even when suspected, it is frequently undiagnosed. When it is diagnosed, it often goes untreated. When it is treated, it is seldom treated optimally” (Shames and Shames, 2002). How true are his words. Almost everyone who walks into my office complains of fatigue, low energy, depression, weight gain or the inability to lose unwanted weight – all symptoms of suboptimal thyroid function. Many times I have found myself scratching my head and asking myself, “What is going on here?” and “Why is it that almost every patient I’ve seen in seven years of practice has something going on with his or her thyroid function?”
Thyroid Hormones 101
Thyroid Stimulating Hormone (TSH) measurement is the blood test most commonly used by conventional doctors to screen for thyroid dysfunction. The “normal” range varies from lab to lab; the most common ranges are 0.3 to 5.5 μIU/ml, with some labs using the newer values of 0.3 to 3.0 μIU/ml established by the American Association of Clinical Endocrinologists (AACE) in 2002. However, if we focus only on TSH we may be missing vital clues in the patient’s thyroid hormone function. Unfortunately, too often, if TSH measures within the “normal” range, the patient’s complaints are dismissed and left untreated. TSH is a very poor indicator of thyroid hormone function and should never be used alone to evaluate or monitor it. Comprehensive testing should be performed that includes free levels of thyroid hormones, free thyroxine (fT4) and free triiodothyronine (fT3) to evaluate peripheral conversion problems. Antithyroperoxidase antibodies (TPO-Ab) should be measured to rule in/out autoimmune thyroid disease (i.e., Hashimoto’s). Many patients come to me saying that their doctor said their thyroid tests were all normal; however, upon further scrutiny of their labs and with comprehensive testing I have seldom found this to be the case.
The first mistake some physicians make upon finding that their patient is hypothyroid is to immediately put them on thyroid hormone replacement therapy. Thyroid hormone, be it synthetic or glandular, will increase patients’ metabolic rate and some will initially report feeling better with increases in energy and less fatigue. However, in many patients this is often a transient effect, and the benefits they initially reported may begin to fade. This most likely occurs when doctors only treat the symptoms with medication and do not address the underlying cause. I have witnessed time and again that, when medication for hypothyroidism is the sole therapy, many patients will eventually complain that they no longer feel well. The old symptoms of hypothyroidism tend to creep back in and they feel they are back where they started, or even worse off than before they were given the medication.
When adrenal function has been weakened by chronic stress, poor diet and environmental toxins, thyroid medication may further stress the system by accelerating the metabolic rate, causing the patient to feel even more fatigue and exhaustion. I believe that it is a mistake to prematurely rush in and give thyroid hormone for patients who are hypothyroid, as it may cause them to crash and burn. Before treating with thyroid hormone, it is absolutely necessary to get a clearer picture of the patient and ask how and why the patient got here in the first place.
Tolle Causam
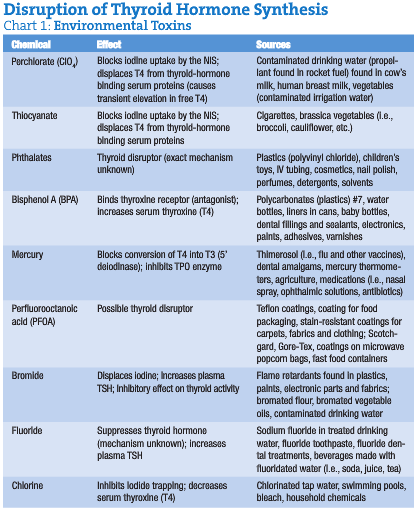
As with most chronic disease, thyroid dysfunction tends to be multifactorial; very seldom is there one single initiating cause. Stress, aging, cigarette smoking, insulin resistance, genetics, nutritional deficiencies/imbalances, extreme dieting and/or starvation, iodine excess and/or deficiency, goitrogens, poor digestion/absorption and dysbiosis are contributing factors (Helmreich et al., 2006; Peeters, 2008; Bufalo et al., 2008; Steinmaus et al., 2007; Rezzonico et al., 2008; Panicker et al., 2008; Ban and Tomer, 2005; Zimmermann, 2007; Moncayo et al., 2008; Kucharzewski et al., 2003; Gärtner et al., 2002; Boelen et al., 2008; Markou et al., 2008; Rao and Lakshmy, 1995; Lauritano et al., 2007). Toxins from the environment, such as pesticides, herbicides, PCBs, dioxins and heavy metals such as mercury, are known to disrupt the delicate balance of thyroid hormone (Abdelouahab et al., 2008; Köhrle, 2008). Perchlorate and thiocyanates (Sanchez et al., 2007; Laurberg et al., 2002) have been shown to block the sodium-iodide symporter (NIS) and prevent the uptake of iodine into the thyroid gland (De Groef et al., 2006; Hershman, 2005). Exogenous hormones such as xenoestrogens, birth control pills and hormone replacement therapy have also been shown to interfere with thyroid function (Gray Jr., 1998). Halogens such as bromine, chlorine and fluorine may interfere with the NIS and also prevent iodine uptake into the thyroid gland (Darnerud, 2008; Maervoet et al., 2007; Pathak et al., 2008; Antignac et al., 2008). More recently, plastics (i.e., phthalates, bisphenol A) have been in the news and are being implicated in hormone disruption and thyroid dysfunction (Huang et al., 2007; Meeker et al., 2007; Kaneko et al., 2008).
 Environmental Effects on Thyroid Function
Environmental Effects on Thyroid Function
Various environmental chemicals can disrupt thyroid hormones and the thyroid’s function (Brown, 2003; see Chart 1). It is the bioaccumulation of these chemicals over the years in our tissues that creates hormone disruption and imbalance.
The European Journal of Endocrinology reported in 2006: “Several groups of chemicals have potential for thyroid disruption. There is substantial evidence that polychlorinated biphenyls, dioxins and furans cause hypothyroidism in exposed animals and that environmentally occurring doses affect human thyroid homeostasis. Similarly, flame retardants reduce peripheral thyroid hormone (TH) levels in rodents, but human studies are scarce. Studies also indicate thyroid-disruptive properties of phthalates, but the effect of certain phthalates seems to be stimulative on TH production, contrary to most other groups of chemicals. Thyroid disruption may be caused by a variety of mechanisms, as different chemicals interfere with the hypothalamic-pituitary-thyroid axis at different levels. Mechanisms of action may involve the sodium-iodide symporter, thyroid peroxidase enzyme, receptors for THs or TSH, transport proteins or cellular uptake mechanisms. The peripheral metabolism of the THs can be affected through effects on iodothyronine deiodinases or hepatic enzymes. Even small changes in thyroid homeostasis may adversely affect human health, and especially fetal neurological development may be vulnerable” (Boas et al., 2006).
Every man, woman and child on Earth has been found to harbor these toxins in their tissues. Even more disturbing is the fact that cord blood of newborn babies was found to contain these harmful compounds, exposing neonates to their damaging effects even before they are born (Maervoet et al., 2007; Iijima et al., 2007; Pathak et al., 2008; Antignac et al., 2008).
I have often pondered that what we may consider dysfunction may not be dysfunction at all, and that the slowing of the metabolic rate in hypothyroidism may actually be an important mechanism that protects the body from undue harm posed by chronic exposure to harmful substances in our environment. Down-regulation of the hypothalamus-pituitary-thyroid (HPT) axis may be a protective mechanism to conserve energy in times of stress. Chronic, low-dose exposure to environmental chemicals most certainly must be considered a stressor to all life on this planet. One just has to glance at Chart 1 to get an idea of the magnitude of the problem. Based on this evidence, metabolism most likely slows to protect the organism from the toxic effects of its internal and external environment. It is interesting to note that hyperthyroidism often precedes the hypothyroid state. A common trigger of hyperthyroidism is excessive stress. When you ask a hyperthyroid patient what was occurring in his or her life before they were diagnosed with hyperthyroidism, many of them will tell you of a tragic event or that a period of severe stress precipitated their diagnosis. In hyperthyroidism the metabolic rate accelerates and metabolism is upregulated. At this rate the cells are rapidly metabolizing toxins. I propose it is reasonable to consider that if the liver (phase II) is unable to keep up with detoxifying this large metabolic load, toxic metabolites will build up in the bloodstream and begin to interfere with metabolic functions. Therefore, the metabolic rate slows down in an attempt to reduce the rate of toxic metabolites that are building up, thereby leading to a hypothyroid state in order to protect itself. Obviously, this scenario is theoretical; nevertheless, the body in its own mysterious wisdom always strives to do the right thing. Could this so-called disease actually be the body’s way of attempting to correct the problem? It is our job as NDs to identify and remove the barriers to healing.
 Uncovering the Underlying Cause
Uncovering the Underlying Cause
Ask yourself: Why do patients have thyroid dysfunction in the first place? Evaluate their lifestyle; help them build a solid foundation on which they can begin to heal. Look at their diet and nutritional status and work with them to optimize it; find out if they are getting enough high-quality sleep, and if they are not, help them to achieve it. Ask questions to find out how they are coping with the stressors in their lives. What is upsetting the balance, what are the barriers to healing? Ask yourself not “are they toxic?” but “how toxic are they?” and develop a comprehensive detoxification plan based on their biochemical individuality.
As you can see, cleaning up thyroid patients’ internal and external environment becomes a very important matter in helping them to improve thyroid function and begin to heal. There are simple things we can teach our patients to do, such as avoiding polycarbonate plastics (i.e., phthalates, Bisphenol A) and drinking reverse-osmosis purified water instead of tap water to avoid contaminants such as perchlorate, fluoride, bromide, chloride and mercury.
Other important steps should include evaluating the patient’s total body burden. Look for sources of exposure to heavy metals such as mercury (i.e., dental amalgams, dietary fish, vaccinations) and xenoestrogen exposure such as hormone replacement therapy, birth control pills and excessive exposure to estrogen mimics (pesticides, plastics, cosmetics, hormones in animal products, etc.).
 Detoxification
Detoxification
Total body burden makes detoxification a very important matter. However, placing someone on a cleanse or water fast is not necessarily the best step to take. There is evidence that when someone loses weight, the toxins that are stored in the adipose tissue will be released into the bloodstream. The circulating toxins disrupt endocrine function and interfere with thyroid function. Proper and safe methods of detoxification are essential for the patient and should include nutrients (i.e., glutathione, sulfur amino acids, etc.) that have been shown to support phase I and phase II liver detoxification. Detoxification powders may be used and are appropriate for detoxifying most patients. Use of saunas and hydrotherapy may also be appropriate for the patient to enhance the detoxification process. It is important to evaluate patients’ needs and create a program just for them.
Diet and Nutrition
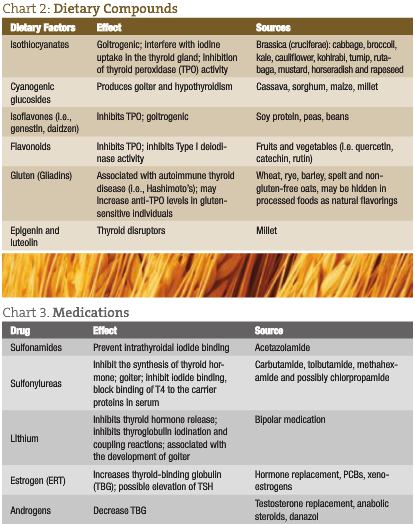
Encourage an organic, whole foods diet rich in plant foods (i.e., phytochemicals and minerals). Optimizing nutrition through diet and nutritional supplements is necessary to lay down the foundation for healing. It has been found that certain foods may interfere with thyroid function – especially gluten found in wheat, rye and barley.
Goitrogenic properties found in the brassica (cruciferae) family of vegetables (i.e., broccoli, collard greens, mustard greens, arugula, Brussels sprouts and kale) have been shown to impede thyroid function. Nevertheless, it is not advised to avoid these foods, because they have been shown to help the body to detoxify; simply recommend that patients do not eat too many in their raw state. Cooking has been shown to deactivate the goitrogenic properties associated with this family of vegetables. Isoflavones, found in beans, especially soy, have also been shown to impede thyroid function. However, studies have found that soy did not cause problems in individuals with adequate iodine intake (Bruce et al., 2003; Messina and Redmond, 2006).
Vitamin D status should be evaluated in individuals with thyroid dysfunction, as it is involved in helping T3 bind to nuclear receptors (Darling et al., 1991). It has been suggested that optimal 25-hydroxy vitamin D3 levels should be between 50 and 80nmol/L in the blood (Dawson-Hughes et al., 2005). Food sources of vitamin D are wild-caught, cold-water fish such as salmon, mackerel, sardines and herring.
Other nutrients such as selenium (Moncayo et al., 2008), zinc (Moncayo et al., 2008; Kucharzewski et al., 2003) and vitamin A (Zimmermann, 2007) are also important for thyroid health.
Sleep, Exercise and Stress Management
Address sleep quantity and quality, amount of exercise (too little, too much), and stress levels and coping skills in all thyroid patients. It is interesting to note that the thyroid and adrenal glands cannot function without each other, and to address only the thyroid without addressing adrenal health is not good medicine (see the accompanying sidebar, Steps to Begin Optimizing Thyroid Function).
As you can see, there is not one simple answer that fully corrects the thyroid epidemic we are seeing in this country. Nevertheless, addressing the underlying causes and removing the barriers to healing provide the best opportunity for healing patients with thyroid disorders.
 Connie Sanchez, ND received her doctorate of naturopathic medicine from NCNM in 2001. With more than 20 years experience in the field of nutrition and fitness, she is a popular speaker on topics such as thyroid health and autoimmunity. In 2005 she co-hosted the radio talk show ‘The Wellness Forum,’ and is the former director of nutrition for Vitamin Cottage Natural Grocers. Dr. Sanchez is currently in private practice at The Center for Health in Lakewood, Colo.
Connie Sanchez, ND received her doctorate of naturopathic medicine from NCNM in 2001. With more than 20 years experience in the field of nutrition and fitness, she is a popular speaker on topics such as thyroid health and autoimmunity. In 2005 she co-hosted the radio talk show ‘The Wellness Forum,’ and is the former director of nutrition for Vitamin Cottage Natural Grocers. Dr. Sanchez is currently in private practice at The Center for Health in Lakewood, Colo.
References
American Association of Clinical Endocrinologists (AACE): www.thyroidawareness.com/faqs.php, /top10.php
Shames R and Shames K: Thyroid Power: Ten Steps to Total Health. New York, 2002, Harper Collins.
American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Evaluation and Treatment of Hyperthyroidism and Hypothyroidism (2006 amended version), Endocrine Practice Nov/Dec 8(6), 2002.
Helmreich DL et al: Peripheral triiodothyronine (T(3)) levels during escapable and inescapable footshock, Physiol Behav Jan 30;87(1):114-9, 2006.
Peeters RP: Thyroid hormones and aging, Hormones (Athens) Jan-Mar;7(1):28-35, 2008.
Bufalo NE et al: Genetic polymorphisms associated with cigarette smoking and the risk of Graves’ disease, Clin Endocrinol (Oxf) Jun;68(6):982-7, 2008.
Steinmaus C et al: Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001-2002 national health and nutrition examination survey, Environ Health Perspect Sep;115(9):1333-8, 2007.
Rezzonico J et al: Introducing the thyroid gland as another victim of the insulin resistance syndrome, Thyroid Apr;18(4):461-4, 2008.
Panicker V et al: A common variation in Deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and trii[o]dothyronine, J Clin Endocrinol Metab May 20, 2008 [Epub ahead of print].
Ban Y and Tomer Y: Genetic susceptibility in thyroid autoimmunity, Pediatr Endocrinol Rev Sep;3(1):20-32, 2005.
Zimmermann MB: Interactions of vitamin A and iodine deficiencies: effects on the pituitary-thyroid axis, Int J Vitam Nutr Res May;77(3):236-40, 2007.
Moncayo R et al: The role of selenium, vitamin C, and zinc in benign thyroid diseases and of selenium in malignant thyroid diseases: low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma, BMC Endocr Disord Jan 25;8:2, 2008.
Kucharzewski M et al: Copper, zinc, and selenium in whole blood and thyroid tissue of people with various thyroid diseases, Biol Trace Elem Res Summer;93(1-3):9-18, 2003.
Gärtner R et al: Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations, J Clin Endocrinol Metab Apr;87(4):1687-91, 2002.
Boelen A et al: Fasting-induced changes in the hypothalamus-pituitary-thyroid axis, Thyroid Feb;18(2):123-9, 2008.
Markou KB et al: Treating iodine deficiency: long-term effects of iodine repletion on growth and pubertal development in school-age children, Thyroid Apr;18(4):449-54, 2008.
Rao PS and Lakshmy R: Role of goitrogens in iodine deficiency disorders & brain development, Indian J Med Res Nov;102:223-6, 1995.
Lauritano EC et al: Association between hypothyroidism and small intestinal bacterial overgrowth, J Clin Endocrinol Metab Nov;92(11):4180-4, 2007.
Abdelouahab N et al: Gender differences in the effects of organochlorines, mercury, and lead on thyroid hormone levels in lakeside communities of Quebec (Canada), Environ Res Jul;107(3):380-92, 2008.
Köhrle J: Environment and endocrinology: The case of thyroidology, Ann Endocrinol (Paris) Apr;69(2):116-22, 2008.
Sanchez CA et al: Perchlorate, thiocyanate, and nitrate in edible cole crops (Brassica sp.) produced in the lower Colorado River region, Bull Environ Contam Toxicol Dec;79(6):655-9, 2007.
Laurberg P et al: Thiocyanate in food and iodine in milk: from domestic animal feeding to improved understanding of cretinism, Thyroid Oct;12(10):897-902, 2002.
De Groef B et al: Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects, Eur J Endocrinol Jul;155(1):17-25, 2006.
Hershman JM: Perchlorate and thyroid function: what are the environmental issues?, Thyroid May;15(5):427-31, 2005.
Gray LE Jr: Tiered screening and testing strategy for xenoestrogens and antiandrogens, Toxicol Lett Dec 28;102-103:677-80, 1998.
Darnerud PO: Brominated flame retardants as possible endocrine disrupters, Int J Androl Apr;31(2):152-60, 2008.
Maervoet J et al: Association of thyroid hormone concentrations with levels of organochlorine compounds in cord blood of neonates, Environ Health Perspect Dec;115(12):1780-6, 2007.
Pathak R et al: Endosulfan and other organochlorine pesticide residues in maternal and cord blood in North Indian population, Bull Environ Contam Toxicol May 17, 2008 [Epub ahead of print].
Antignac JP et al: Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data, Mol Nutr Food Res Feb;52(2):258-65, 2008.
Huang PC et al: Associations between urinary phthalate monoesters and thyroid hormones in pregnant women, Hum Reprod Oct;22(10):2715-22, 2007.
Meeker JD et al: Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men, Environ Health Perspect Jul;115(7):1029-34, 2007.
Kaneko M et al: Bisphenol A acts differently from and independently of thyroid hormone in suppressing thyrotropin release from the bullfrog pituitary, Gen Comp Endocrinol Feb 1;155(3):574-80, 2008.
Brown VJ: Disrupting a delicate balance: environmental effects on the thyroid, Environ Health Perspect Sept; 111(12): A642-9, 2003.
Boas M et al: Environmental chemicals and thyroid function, Eur J Endocrinol May;154(5):599-611, 2006.
Iijima K et al: Cadmium, lead, and selenium in cord blood and thyroid hormone status of newborns, Biol Trace Elem Res Oct;119(1):10-8, 2007.
Zoeller RT: Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals?, Mol Cell Endocrinol Oct 20;242(1-2):10-15, 2005.
Bruce B et al: Isoflavone supplements do not affect thyroid function in iodine-replete postmenopausal women, J Med Food Winter;6(4):309-16, 2003.
Messina M and Redmond G: Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature, Thyroid Mar;16(3):249-58, 2006.
Darling DS et al: 3,5,3’-triiodothyronine (T3) receptor-auxiliary protein (TRAP) binds DNA and forms heterodimers with the T3 receptor, Mol Endocrinol 5(1):73-84, 1991.
Dawson-Hughes B et al: Estimates of optimal vitamin D status, Osteoporosis Int Jul;16(7):713-6, 2005.