Autoimmune Thyroid Disease: Treating With Nutrients and Botanicals
Michaël Friedman, ND
Normally, the immune system provides the body with a defense against foreign substances by producing antibodies that direct blood cells to destroy the unwanted invader. However, sometimes the immune system malfunctions and attacks the body’s own cells and tissues; this is known as autoimmune disease. The reason for this dysfunction is not clearly understood, however many environmental and endogenous factors have been associated with autoimmune disease.
Autoimmune disorders often affect blood vessels and cells, muscle and joint tissue, and endocrine glands. The thyroid is the most commonly affected organ1 and is vulnerable to autoimmune disease due to its complexity. The thyroid is touted as one of the most important glands in the body, as it produces thyroxine (T4), triiodothyronine (T3), and calcitonin. Thyroid hormones regulate metabolism (including utilization of carbohyd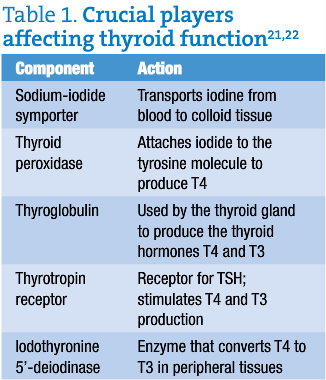 rates, proteins, and fats), which in turn directs cellular activity and stimulates organs including the heart and brain.
rates, proteins, and fats), which in turn directs cellular activity and stimulates organs including the heart and brain.
Prevalence of Autoimmune Thyroiditis
Graves’ disease and Hashimoto’s disease are both autoimmune diseases of the thyroid. Hyperthyroidism is usually characteristic of Graves’ disease, while Hashimoto’s disease most often features hypothyroidism. In iodine-sufficient parts of the world, hypothyroidism is most often caused by autoimmune thyroiditis (Hashimoto’s thyroiditis), whereas iodine deficiency is the main cause of hypothyroidism worldwide.2 Both Hashimoto’s and Graves’ disease are characterized by T and B cell infiltration of the thyroid, production of thyroid autoantibodies, and abnormal thyroid function.
In Graves’ disease and Hashimoto’s disease, B and T lymphocyte-mediated autoimmunity is directed against thyroglobulin (Tg), thyroid peroxidase (TPO), sodium-iodide symporter (NIS) and thyrotropin receptor (TSH-R). All are key to proper thyroid function and thyroid hormone homeostasis (table 1).
The presence of anti-TPO and anti-Tg antibodies (TgAb) in the blood can be used to diagnose underlying autoimmunity in the absence of clinical symptoms.1 Elevation of these autoimmune antibodies may precede diagnosis of clinical (symptomatic) autoimmune disease by 2 to 7 years.3 Although 90% of patients with Hashimoto’s disease have elevated circulating TgAb, the presence of high TgAb, alone, is not sufficient to make a diagnosis, as this abnormality is also seen in patients with other autoimmune disorders such as rheumatoid arthritis and type I diabetes.4
Possible Contributing Factors
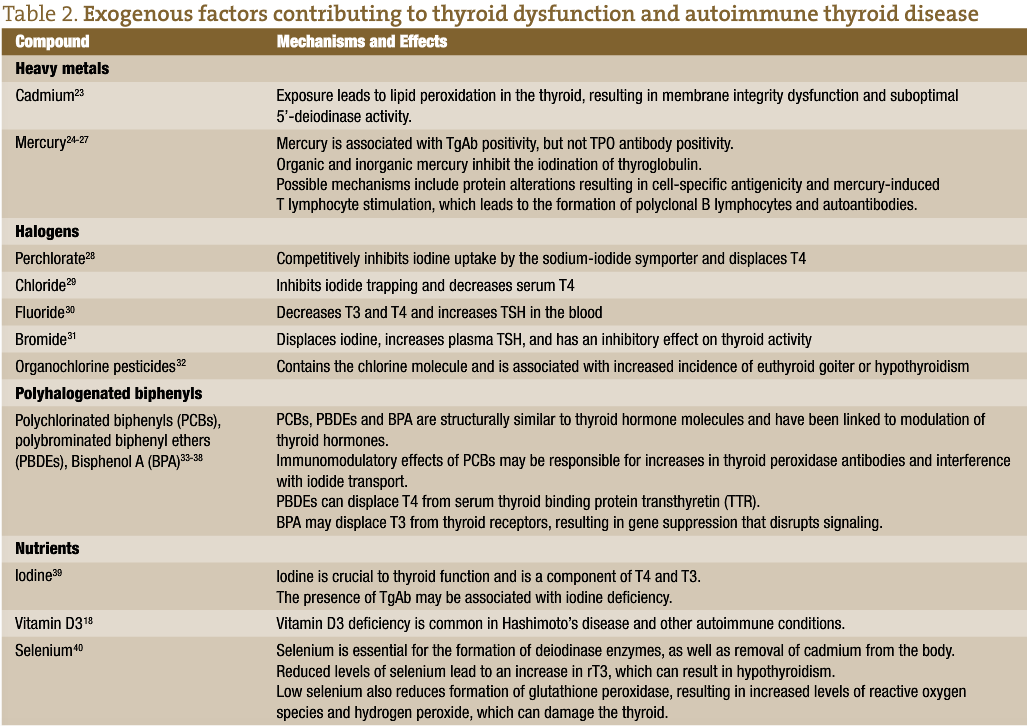
Interaction between genetic variants that promote susceptibility and environmental factors are believed to result in autoimmune thyroiditis. Etiological factors associated with autoimmune thyroid disease include infections or other diseases, gender, virus, dietary iodine intake, and environmental factors, among others. Genetic predisposition may play a role in autoimmune thyroiditis. Onset of the disease is triggered by environmental factors including heavy metals, halogens, pesticides, polyhalogenated biphenyls and nutrient deficiencies, among others.5 Adverse effects due to halogens and other chemical contaminants are generally seen only with exposure to high amounts of these compounds and usually only with a mixture of chemicals, not to individual chemicals alone.6 Other factors such as radiation,7 infections,8 medications,9 nutritional deficiencies,10-12 industrial pollutants, coal pollution, motor vehicle emissions, and agricultural fungicides have also been suggested to play a role in thyroid dysfunction and autoimmune thyroid disease.5,13 Deficiencies in nutrients such as selenium, iodine, iron, zinc, vitamin A, and vitamin B12 have been associated with thyroid dysfunction.10-12,14 Iodine is essential for thyroid hormone synthesis. Some investigators have suggested that iodine intake in excess of 1100 mcg per day in adults can increase the risk of thyroid dysfunction in susceptible individuals.15 Others, however, have concluded that while areas with iodine sufficiency appear to have higher incidences of hypothyroidism, there is no consistent evidence that excessive iodine levels contribute to additional risk.1,16 The mechanisms by which these various compounds affect thyroid function, thyroid hormone synthesis, and immune function differ by compound (table 2).
Herbs and Nutrients for Autoimmune Thyroid Disorders
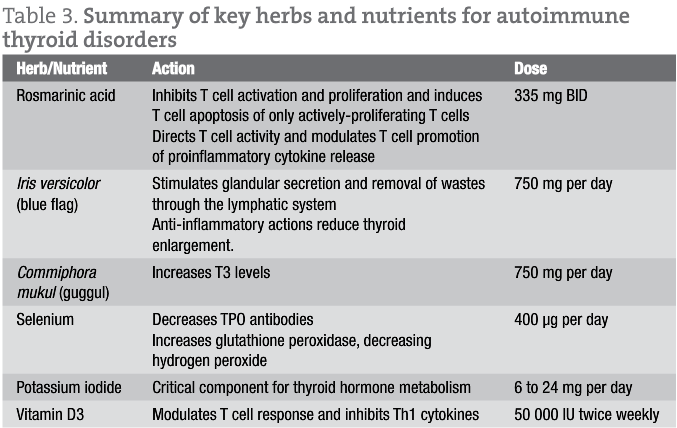
Drug therapy is often recommended for thyroid disorders. Standard medical protocol for Hashimoto’s disease is thyroid hormone replacement, since hypothyroidism is the usual presentation. For Graves’ disease, which presents as hyperthyroidism, anti-thyroid drugs, surgical removal (partial or full) of the thyroid gland, and/or use of radioactive iodine (131I) is usually recommended. Natural products recommended for autoimmune thyroid disorders include vitamin D3, rosmarinic acid, blue flag, guggul, selenium, and possibly iodide supplementation.17
Suggested Protocols
Vitamin D3 supplementation is suggested for both autoimmune hypothyroid and hyperthyroid disease, due to the immunomodulatory effects of vitamin D3 and the association of vitamin D3 deficiency with many autoimmune diseases.18 The recommended dose of vitamin D3 is 50 000 iu, up to 2 times per week. When treating patients with vitamin D3, blood concentrations of 25(hydroxy)vitamin D should be monitored to ensure that sufficiency is achieved (>40 ng/mL) and that vitamin D-induced hypercalciuria (>150 ng/mL) does not occur.19
In addition to vitamin D3 supplementation, the following protocols are recommended:
- For autoimmune hypothyroidism: Iris versicolor (blue flag), 750 mg per day, also Commiphora mukul (guggul), 750 mg per day, for their anti-inflammatory effects. For general thyroid support: iodine, 6-24 mg per day,20 and selenium, 400 mcg per day.
- For autoimmune hyperthyroidism: Rosmarinic acid, 335 mg BID, for its immunomodulatory effects. For general thyroid support, clinical experience suggests: iodine, 6 mg per day, and selenium, 400 mcg per day.
Further details of the specific herbs and nutrients and their effects on the immune response and thyroid hormones are provided in table 3.
Conclusion
Vitamin D3, rosmarinic acid, guggul, blue flag, selenium, and iodine are indicated for the treatment of autoimmune thyroid disorders. Each of these compounds has varied mechanisms of action supporting thyroid hormone homeostasis. For example, vitamin D3 and rosmarinic acid play a role in T cell modulation and cytokine response. It is also imperative that patients be counseled on reducing stress, improving digestion and absorption, and ensuring proper nutrition including the avoidance of foods known to interfere with proper thyroid function. It is also important for patients with autoimmune thyroid disease to incorporate aerobic exercise into their lifestyle, as it induces 5′-deiodinase, the enzyme that stimulates peripheral production of active T3 from T4. When treated appropriately, restoration of normal thyroid function in early stages of autoimmune thyroid disease can be achieved in patients with autoimmune thyroid disease.
 Michaël Friedman, ND is the Executive Director of the non-profit Association for the Advancement of Restorative Medicine (AARM) a professional society for licensed physicians whose goal is to offer high level education and promote excellence in clinical medicine for its members and conference attendees. He is the editor and chief of the affiliated Journal of Restorative Medicine.
Michaël Friedman, ND is the Executive Director of the non-profit Association for the Advancement of Restorative Medicine (AARM) a professional society for licensed physicians whose goal is to offer high level education and promote excellence in clinical medicine for its members and conference attendees. He is the editor and chief of the affiliated Journal of Restorative Medicine.
References
- McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42:252-265.
- Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull 2011;99:39-51.
- Hutfless S, Matos P, Talor MV, et al. Significance of prediagnostic thyroid antibodies in women with autoimmune thyroid disease. J Clin Endocrinol Metab. 2011;96:E1466-E1471.
- Gallagher CM, Meliker JR. Mercury and thyroid autoantibodies in U.S. women, NHANES 2007-2008. Environ Int. 2012;40:39-43.
- Duntas LH. Environmental factors and autoimmune thyroiditis. Nature Clinical Practice Endocrinology & Metabolism [serial online]. August 2008;4(8):454-460.
- Porter WP, Jaeger JW, Carlson IH. Endocrine, immune, and behavioral effects of aldicarb (carbamate), atrazine (triazine) and nitrate (fertilizer) mixtures at groundwater concentrations. Toxicol Ind Health. 1999;15:133-150.
- Mangano JJ. A post-Chernobyl rise in thyroid cancer in Connecticut, USA. Eur J Cancer. Prev 1996;5:75-81.
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 10th ed. Philadelphia, PA: WB Saunders; 2003.
- Dong BJ. How medications affect thyroid function. West J Med. 2000;172:102-106.
- Delange F. The disorders induced by iodine deficiency. Thyroid. 1994;4:107-128.
- Pizzulli A, Ranjbar A. Selenium deficiency and hypothyroidism: a new etiology in the differential diagnosis of hypothyroidism in children. Biol Trace Elem Res. 2000;77:199-208.
- Watts DL. The nutritional relationships of the thyroid. Journal of Orthomolecular Medicine. 1989;4:165-169.
- Safran M, Paul TL, Roti E, Braverman LE. Environmental factors affecting autoimmune thyroid disease. Endocrinol Metab Clin North Am. 1987;16:327-342.
- Stangl GI, Schwarz FJ, Kirchgessner M. Cobalt deficiency effects on trace elements, hormones and enzymes involved in energy metabolism of cattle. Int J Vitam Nutr Res. 1999;69:120-126.
- Leung AM, Braverman LE. Iodine-induced thyroid dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19(5):414-9.
- Abraham GE. Facts about Iodine and Autoimmune Thyroiditis. The Original Internist. 2008;15(2): 75-76.
- Friedman M. Fundamentals of Naturopathic Endocrinology: A Complementary And Alternative Medicine Guide. Canadian College of Naturopathic Medicine; 2005.
- Tamer G, Arik S, Tamer I, Coksert D. Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid. 2011;21(8):891-896.
- Watson RR, ed. Handbook of Vitamin D in Human Health: Prevention, Treatment and Toxicity. Wageningen Academic Publishers; 2013.
- Brownstein D. Clinical experience with inorganic, non-radioactive iodine/iodide. The Original Internist. 2005; 12(3):105-108.
- Nussey S, Whitehead S, eds. Endocrinology: An Integrated Approach. Oxford. BIOS Scientific Publishers; 2001.
- Melmed S and Conn MP, eds. Endocrinology: Basic and Clinical Principles. Springer; 2005.
- Gupta P, Kar A. Role of ascorbic acid in cadmium-induced thyroid dysfunction and lipid peroxidation. J Appl Toxicol. 1998;18:317-320.
- Nishida M, Sato K, Kawada J. Differential effects of methylmercuric chloride and mercuric chloride on oxidation and iodination reactions catalyzed by thyroid peroxidase. Biochem Int. 1990;22:369-378.
- Kawada J, Nishida M, Yoshimura Y, Mitani K. Effects of organic and inorganic mercurials on thyroidal functions. J Pharmacobiodyn. 1980;3:149-159.
- Pusey CD, Bowman C, Morgan A, Weetman AP, Hartley B, Lockwood CM. Kinetics and pathogenicity of autoantibodies induced by mercuric chloride in the brown Norway rat. Clin Exp Immunol. 1990;81:76-82.
- Powell JJ, Van de Water J, Gershwin ME. Evidence for the role of environmental agents in the initiation or progression of autoimmune conditions. Environ Health Perspect. 1999;107 Suppl 5:667-672.
- Merrill EA, Clewell RA, Gearhart JM et al. PBPK predictions of perchlorate distribution and its effect on thyroid uptake of radioiodide in the male rat. Toxicol Sci. 2003;73:256-269.
- Harrington RM, Shertzer HG, Bercz JP. Effects of chlorine dioxide on thyroid function in the African green monkey and the rat. J Toxicol Environ Health. 1986;19:235-242.
- Wang H, Yang Z, Zhou B, Gao H, Yan X, Wang J. Fluoride-induced thyroid dysfunction in rats: roles of dietary protein and calcium level. Toxicol Ind Health. 2009;25:49-57.
- Allain P, Berre S, Krari N et al. Bromine and thyroid hormone activity. J Clin Pathol. 1993;46:456-458.
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110 Suppl 3:337-348.
- Brouwer A, Morse DC, Lans MC et al. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health. Toxicol Ind Health. 1998;14:59-84.
- Hagmar L, Rylander L, Dyremark E, Klasson-Wehler E, Erfurth EM. Plasma concentrations of persistent organochlorines in relation to thyrotropin and thyroid hormone levels in women. Int Arch Occup Environ Health. 2001;74:184-188.
- Persky V, Turyk M, Anderson HA et al. The effects of PCB exposure and fish consumption on endogenous hormones. Environ Health Perspect. 2001;109:1275-1283.
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118:1444-1449.
- Marsh AB, Bladh LG, Jakobssson E. Synthesis of p-hydroxybromodiphenyl ethers and binding to the thyroid receptor. Organohalogen Compd. 1998;37:305-308.
- Moriyama K, Tagami T, Akamizu T et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185-5190.
- Andersen S, Iversen F, Terpling S, Pedersen KM, Gustenhoff P, Laurberg P. Iodine deficiency influences thyroid autoimmunity in old age–a comparative population-based study. Maturitas. 2012;71(1):39-43.
- Negro R. Selenium and thyroid autoimmunity. Biologics. 2008;2(2):265-273.