Teerawong Kasiolarn, ND, MSAc, LAc
Tolle Totum
According to the Heart Foundation and the Centers for Disease Control and Prevention (CDC), heart disease remains the leading cause of death for both men and women in the United States.1 In fact, someone has a heart attack every 34 seconds, and coronary heart disease (CHD) is the most common type of heart disease, which kills more than 370 000 people annually in the United States.1,2 Direct and indirect costs of heart disease have been estimated to be more than $320 billion annually.2 Heart disease is highly preventable and is mainly associated with poor dietary and lifestyle habits, with minimal genetic contribution. Naturopathic medicine can be at the forefront to prevent and address this national dilemma.
Metabolic Syndrome & Heart Disease
The strongest risk factor for developing heart disease is metabolic syndrome (MetSyn).3,4 A gold-standard definition of MetSyn is still unclear, as there are multiple definitions offered by various organizations, including the World Health Organization (WHO), the US National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), the European Group for the Study of Insulin Resistance (EGIR), and the International Diabetes Federation (IDF). Despite there being various definitions of MetSyn, the underlying factors are still agreed upon, including abdominal (central) obesity, atherogenic dyslipidemia (elevated triglycerides and/or low HDL-C), hypertension, and insulin resistance (elevated fasting glucose and/or hyperinsulinemia).3-5
Researchers, however, have determined that WHO-defined MetSyn is a better predictor of coronary calcium than are NCEP ATP III criteria, due to WHO’s insulin resistance requirement.6 Personally, I concur with the WHO’s clinical criteria for MetSyn, which specify hyperinsulinemia (fasting insulin level in the upper quartile among non-diabetic individuals) or hyperglycemia (fasting glucose ≥110 mg/dL), in addition to at least 2 of the following criteria5-7:
- Abdominal Obesity: Waist-to-hip ratio >0.9 in men, and >0.85 in women; or body mass index (BMI) >30 kg/m2
- Dyslipidemia: Triglycerides ≥150 mg/dL; and/or HDL-C <40 mg/dL in men, and <50 mg/dL in women
- Hypertension: Blood pressure (BP) ≥140/90 mm Hg, or taking anti-hypertensive medication(s)
- Microalbuminuria: Urinary albumin secretion rate ≥20 μg/min, or albumin-to-creatinine ratio ≥ 30mg/g
A recent study, published in JAMA in September 2015, revealed that 49-52% of adults aged 20 years and older in the United States either have full-blown diabetes or pre-diabetes.8 Most of these individuals, unfortunately, are not aware of their medical condition. This is an alarming result, since people with prediabetes or diabetes have a much higher risk of having cardiac events during their lifetime.
An exciting piece of news is that the IDF has been working on the Platinum Standard Definition of MetSyn; they are utilizing advanced metabolic measures and taking into account the following criteria4:
- Abnormal body fat distribution: Adipose tissue biomarkers (eg, leptin, adiponectin)
- Atherogenic dyslipidemia: Small LDL particles (ApoB, etc)
- Vascular dysregulation: Endothelial dysfunction biomarkers, microalbuminuria, etc
- Insulin resistance: Fasting insulin/proinsulin levels, elevated free fatty acids, HOMA-IR, etc
- Pro-inflammatory state: hs-CRP, IL-6, TNF-α, etc
- Prothrombotic state: PAI-1, fibrinogen, etc
- Hormonal factors: Pituitary-adrenal axis
Ideally, these advanced biomarkers will be able to identify and detect early signs of insulin resistance or metabolic syndrome. The Standard Lipid Panel does not do a good job of evaluating cardiovascular risk, as 50% of people who have had a heart attack received “normal” cholesterol results.9 A more complete evaluation of individuals for MetSyn and cardiovascular risk assessment is clearly essential, as early interventions can often prevent or reverse the progression of heart disease.
This article consists of 2 parts. Part 1 of the article provides an overview of MetSyn and heart disease, introduces a naturopathic case study, and discusses the patient’s comprehensive cardiometabolic laboratory tests and results. Part 2 (upcoming) will delve into the comprehensive naturopathic treatment plan that can resolve MetSyn, and provides follow-up lab results of the same patient following naturopathic treatment.
Naturopathic Case Study
Brief Medical History
A 36-year-old Asian male came to see me for an initial naturopathic consult, with the chief complaint of fatigue for a few years. The patient had also been obese for several years, his weight having steadily crept up over the past 5 years due to physical inactivity and poor eating habits. He was married and had 2 young children, was self-employed, and had been working long hours with moderate stress for several years. He wanted to understand the underlying causes of his fatigue other than working too much. The patient reported sleeping well, getting 7 hours of sleep per night. He had not had an annual physical exam for over 10 years.
The patient had a strong family history of diabetes and heart disease. In fact, his mother was recently admitted to a hospital due to a heart attack, and fortunately survived the event. The patient expressed that he would like to prevent any future cardiac events if possible. He reported feeling motivated to change his lifestyle, since he would like to live a long, healthy life for his wife and his 2 young children. He reported having no chronic health issues, and took no prescription medications. The only over-the-counter medication he took as needed for seasonal allergies was an anti-histamine. The patient denied any alcohol intake or smoking of cigarettes. He had not been taking any nutritional supplements but said he would like advice on nutritional supplementation. He also wanted to get a comprehensive laboratory assessment to find underlying causes of his fatigue, evaluate his cardiovascular risk, address his obesity problem, and thoroughly assess his overall health status. His ultimate goal was to feel well again with good energy, lose weight, and optimize his health.
Physical Exam
Since naturopathic doctors are not licensed in Virginia, I referred this patient to one of our medical providers in the same medical group for an annual physical exam and my recommended comprehensive lab work. The patient was found to be 170.18 cm (67 in) tall, and weighed 104.3 kg (239 lb, BMI 36.0 kg/m2). His overall examination was unremarkable, other than being classified as Class II Obesity with significant central obesity (waist circumference 36.5 in). A series of blood tests (listed below) were performed during this office visit, to rule out metabolic syndrome, nutritional deficiencies, hormonal imbalance (especially thyroid function and sex hormones), genetic risks for heart disease, and to thoroughly assess cardiovascular risk biomarkers and overall health status.
Laboratory Evaluation & Discussion
The patient came to see me 2 weeks after the blood draw for a second naturopathic visit in order to review the lab test results and the naturopathic treatment plan. The information below shows the initial lab results from his annual physical visit.
Complete Blood Count (CBC)
The patient’s CBC results were unremarkable.
Comprehensive Metabolic Panel (CMP)
The patient’s electrolytes, kidney function tests, and proteins were unremarkable.

Figure 1. Fasting Glucose
His fasting glucose (Figure 1) was 94 mg/dL. Even though the lab labeled this level “optimal,” I considered it “borderline-high.” This is because of a 22-year prospective study10 that found that men with fasting blood glucose >85 mg/dL had a significantly increased risk of dying from cardiovascular disease (CVD).10 As a result, my optimal level for a fasting glucose level ranges from 70 to 85 mg/dL. In addition, a fasting glucose test is simply a snapshot of blood glucose at a specific time of day while fasting (with water only) over 8-12 hours. Hence, other, more advanced blood sugar biomarkers should ideally be measured as well for a comprehensive overview of patient’s blood glucose status, including Hemoglobin A1c (HgbA1C), Fructosamine, Fasting Insulin, and Adiponectin. These biomarkers will be discussed in the Comprehensive Diabetes Panel section.
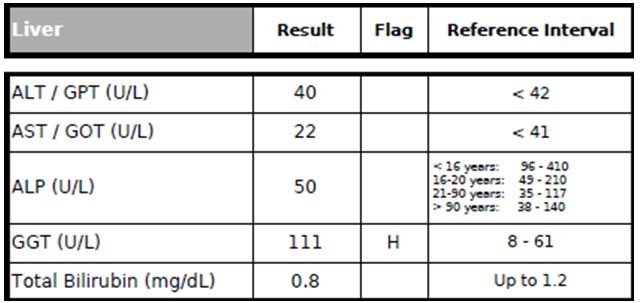
Figure 2. Liver Panel
The patient’s AST, ALP, and total bilirubin (Figure 2) were unremarkable.
However, his ALT (alanine-transaminase) level was borderline-high at 40 U/L, suggesting that he might have an underlying non-alcoholic fatty liver disease (NAFLD) due to his obesity issue. (He denied drinking any alcohol beverages.) The ALT level should be <30 U/L, but ideally <20 U/L.11,12 Researchers found that even when ALT was <30 U/L, subjects whose ALT was in the highest ALT (upper 20s range) had a 1.878-times-higher risk of developing MetSyn than those with ALT in the lowest quartile (lower teens).11
The patient’s GGT (gamma-glutamyltransferase) level was elevated at 111 U/L (almost twice the upper limit). Serum GGT has long been utilized clinically as a biomarker for excessive alcohol consumption or liver disturbances.13 However, GGT has recently been shown to also be a useful biomarker for inflammation and oxidative stress as well as the development of CVD, hypertension, stroke, type 2 diabetes, and their complications, independent of alcohol consumption.13 Moreover, in prospective studies, elevated levels of ALT and GGT have been shown to predict and increase risk of CVD events.11
Standard Lipid Panel
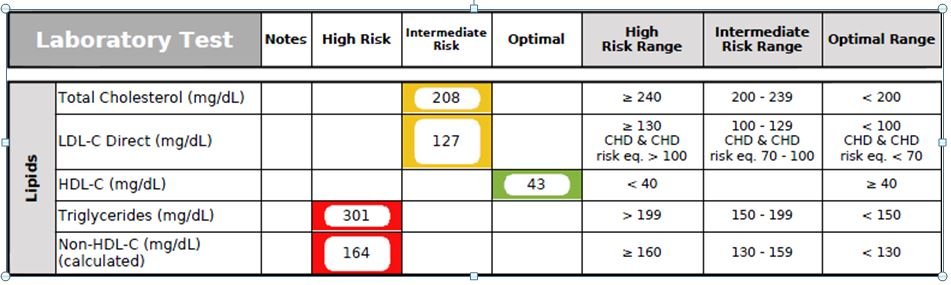
Figure 3. Standard Lipid Panel
The patient’s Total Cholesterol and LDL-C Direct levels (Figure 3) were mildly elevated at 208 mg/dL and 127 mg/dL, respectively. His HDL-C was borderline-low at 43 mg/dL. His Triglycerides and Non-HDL-C levels were highly elevated at 301 mg/dL and 164 mg/dL, respectively. According to the Framingham Heart Study, the patient’s chance of having a myocardial infarction (MI) within the next 10 years was about 3.3%.14 However, as mentioned previously, the Standard Lipid Panel alone is not comprehensive enough to evaluate a patient’s risk for a CV event, since an estimated 50% of patients admitted to hospitals for MI have “normal” LDL-C levels.9 Personally, I have found the Triglyceride (TG) level to be the most clinically relevant biomarker on the Standard Lipid Panel. Optimally, the TG level should be <100 mg/dL.15 The TG/HDL Ratio should be less than 2. In this case, his TG/HDL ratio was highly elevated at 6.98, signifying increased risk of a coronary event.16
Elevated TGs can be due to several causes, including:
- A diet high in refined carbohydrates, animal saturated fats (including dairy products), and trans-fats
- Being physically inactive, overweight, and/or obese
- Other factors, including hypothyroidism, increased alcohol consumption, type 2 diabetes, cigarette smoking, and genetic factors
Advanced Lipoprotein Panel
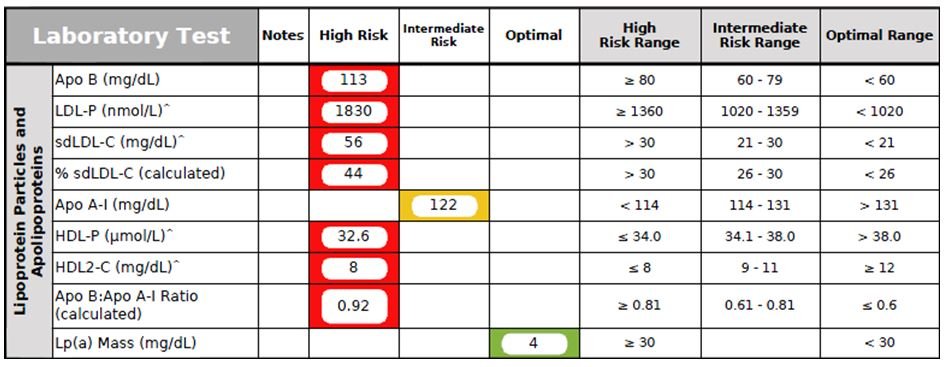
Figure 4. Advanced Lipoprotein Panel
The Advanced Lipoprotein Panel (Figure 4) provides an excellent in-depth CV risk assessment. This patient’s results showed a classic “Atherogenic Lipid Triad”17-19 of elevated TG levels, low healthy cholesterol fractions (Apo A-I, HDL-P, and HDL2-C levels), and high atherogenic cholesterol fractions (Apo B, LDL-P, and sdLDL-C). Fortunately, his Lp(a)-P level (a genetic risk marker for developing coronary artery disease) was normal.
- Apo B (Apolipoprotein B) is the scientifically accepted measurement of atherogenic particle number. Optimally, Apo B should be <70 mg/dL. Several studies identified elevated Apo B concentrations to be highly predictive of CV events.19,20
- HDL2-C, an HDL-C subclass that is larger in size and has a heart-protective effect, is associated with the majority of reverse cholesterol transport and regression of coronary atherosclerosis.18,19,21
- LDL-P (LDL Particle Count) represents the actual number of all-sized LDL particles within a liter of plasma (expressed in nmol/L). An elevated LDL-P level is associated with increased CHD risk, increased carotid intimal medial wall thickness, and has been shown to be a better marker for CV risk assessment than the traditional LDL-C marker.18,19
- sdLDL-C (small-dense LDL-C) is associated with increased atherogenic risk, especially in combination of high TGs and low HDL-C.17-19,21 Increased sdLDL-C has been associated with both the presence and severity of CHD.20 Small-dense LDL particles are more likely to damage artery walls than larger LDL particles.18,21
- Apo A-I (Apolipoprotein A-1), the major protein component of HDL, plays an essential role in reverse cholesterol transport and has an anti-clotting effect on platelets.22 In addition to lowering CVD risk, Apo A-I has been shown to have anti-tumor, anti-inflammatory, and neutralizing effects against endotoxin lipopolysaccharide (LPS).22
- HDL-P (HDL Particle Count) represents all-sized HDL particles within a liter of plasma (mmol/dL). HDL-P has been shown to be a better biomarker than the traditional HDL-C marker.23
- Lp(a)-P (Lipoprotein[a] Particle) is an LDL-like particle that consists of an abnormal protein, apolipoprotein(a), attached to 1 molecule of Apo B100 via a disulfide bond.24 Lp(a) is well recognized as an independent causal risk factor for ischemic CVD, and has been associated with aortic stenosis.24-26 It is usually elevated due to an inherited abnormality.25
Inflammatory & Oxidative Stress Biomarkers
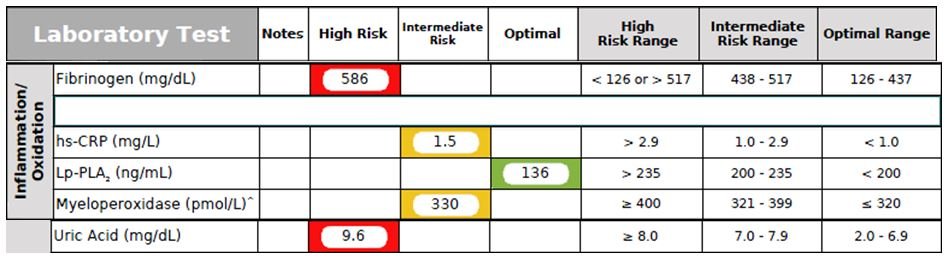
Figure 5. Inflammation/Oxidation Panel
Inflammation and oxidative stress are known contributors to cardiovascular risk. This patient had several elevated markers in this area, reflecting increased CVD risk (Figure 5).
- Fibrinogen is a protein that converts to fibrin during blood clot formation, and is also an acute phase reactant, responding to any inflammatory process including atherosclerotic lesions in the arteries. In this case, the patient was found to have an elevated Fibrinogen level, which enhances platelet adhesion and promotes a prothrombotic state. A high fibrinogen level was shown to be an independent predictor for the development of coronary artery disease (CAD) in the Framingham study.27 In several large prospective studies, fibrinogen has been established as an independent risk factor for CAD.28,29
- hs-CRP (high-sensitivity C-reactive protein) is a highly sensitive assay capable of detecting low levels of CRP. An elevated hs-CRP is currently considered an independent risk factor for atherosclerosis and hypertension.30 The patient’s hs-CRP was mildly elevated.
- Lp-PLA2 (Lipoprotein-associated Phospholipase A2), also known as Platelet-Activating Factor Acetylhydrolase, or PAF-AH), is an enzyme that promotes inflammation and oxidative stress, leading to atherosclerotic lesions.31 An elevated Lp-PLA2 level is a strong risk factor for CHD.32 This patient had a normal level of Lp-PLA2.
- Myeloperoxidase (MPO), an enzyme synthesized and stored within monocytes and polymorphonuclear leukocytes, has been linked, when elevated, to the development of atherosclerotic disease, plaque instability, carotid plaque inflammation, and endothelial dysfunction by limiting nitric oxide bioavailability and by directly consuming nitric oxide.33,34 This patient had an elevated MPO level, which has been shown to be associated with an increased risk of CVD in several studies.35 MPO catalyzes the oxidation of HDL-C, impairing its ability to perform reverse cholesterol transport, and promoting the development of dysfunctional HDL-C.36
- Uric Acid is a product of the metabolic breakdown of purine nucleotides. Purines are found in high concentration in meat products, especially internal organs such as liver and kidney. In this case, the patient’s uric acid level was highly elevated, increasing his risk of developing gout. He denied having any previous gout attacks. Multiple studies have shown that hyperuricemia is also a strong risk factor for CAD, hypertension, and obesity-related MetSyn.37
Genetic Tests
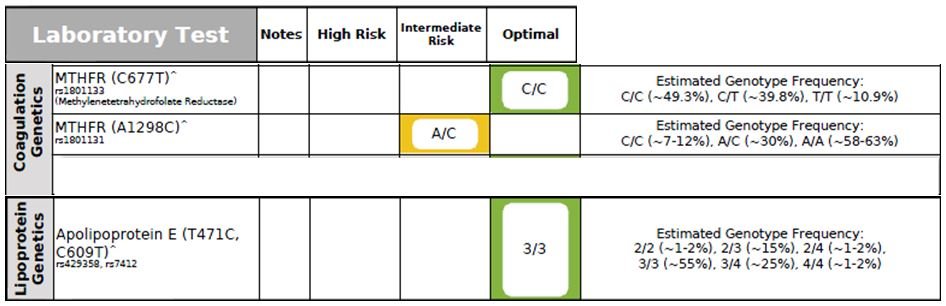
Figure 6. Genetic Markers
C677T & A1298C MTHFR Gene
The methylenetetrahydrofolate reductase (MTHFR) enzyme plays a critical role in maintaining the equilibrium between DNA methylation, DNA synthesis/repair, folate metabolism, and the remethylation of homocysteine (Hcy).38,39 The MTHFR 677 T-allele is associated with reduced enzymatic activity, reduced folate levels, and homocysteinemia (elevated Hcy levels).38,39 Homocysteinemia is a strong risk factor for both CVD; hence, early detection of MTHFR gene mutations, B vitamin status, and Homocysteine level are essential to optimize brain and cardiovascular health.40 The presence of MTHFR gene mutations also increase the risk of thrombosis.38,39
In addition, a large meta-analysis study showed that the MTHFR T-allele increases the risk for Alzheimer’s disease (AD) in the general population, particularly in Asian populations.40 Although the MTHFR 1298 C-allele is associated with decreased enzymatic activity, it has not been linked with homocysteinemia.38,39 Although this patient’s genetic analysis (Figure 6) showed a heterozygous A1298C genotype, his Homocysteine level was elevated; this could be due to borderline-low levels of serum B12 and RBC Folate (discussed below).
Apolipoprotein E (ApoE) Gene
Apo E-containing lipoproteins transport dietary lipids to the tissues for storage, and transport cholesterol and other lipids from the tissues to the liver for excretion; hence, genetic abnormality in the ApoE gene would result in lipid metabolism problems.41 The APOE gene polymorphism has been associated with CVD, hypertension, dyslipidemia, diabetes, neurodegenerative disorders, and MetSyn.37 The APOE gene exists in 3 different forms, including ApoE2, E3, and E4. Epidemiological studies have demonstrated that E4-allele carriers are particularly predisposed to higher cholesterol levels and a higher incidence of CAD mortality,42,43 although E4 carriers without visceral obesity appear to have a lower prevalence of CVD.42 ApoE4 individuals should limit the amount of saturated, trans fat, and alcohol in their diet. ApoE2 individuals are prone to develop diabetes and insulin resistance, so should further limit the amount of carbohydrate and sugar in the diet.44
This patient was found to have the ApoE3/E3 genotype, which is considered normal lipid metabolism. Individuals carrying ApoE3 should eat a portion-controlled, balanced diet that is rich in vegetables, some fruits, and low in sugar.
Nutritional Status Biomarkers
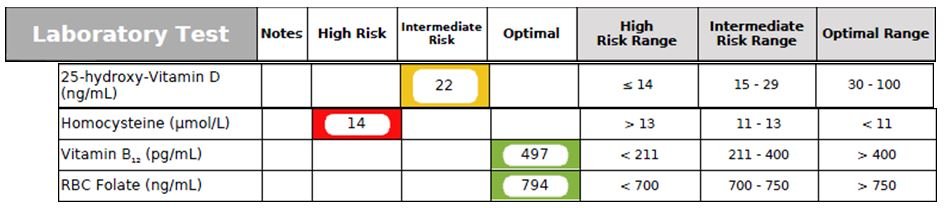
Figure 7. Nutritional Status Biomarkers
- 25-hydroxy-Vitamin D: Vitamin D deficiency is very common due to our modern lifestyle, in which we are mostly living and working indoors, with minimal exposure to sunlight. This patient had a clear vitamin D deficiency (Figure 7). Optimally, I like to see the 25-hydroxy-Vitamin D level in the 60-80 ng/mL range. Vitamin D deficiency is associated with chronic degenerative diseases such as atherosclerosis, hypertension, insulin resistance, diabetes mellitus, MetSyn, osteopenia/osteoporosis, cancers (eg, breast, colon), autoimmune diseases (eg, multiple sclerosis, rheumatoid arthritis, psoriasis), fatigue, muscle pain, dementia, AD, chronic infections, and all-cause mortality.45-47
- Homocysteine (Hcy): (See the MTHFR gene section for a discussion of Hcy). The patient’s Homocysteine level was elevated at 14 mmol/L. An optimal level of Hcy is <8 mmol/L.
- Serum B12 & RBC Folate tests should be checked along with Homocysteine, as B vitamin status has a huge impact on Hcy levels. Optimally, serum B12 should be >800 pg/mL, and RBC Folate should be >1000 ng/mL. The patient’s serum B12 and RBC Folate were both borderline-low. Researchers have found that low B vitamins can increase the risk of developing MetSyn.48 Supplementation of L-methylfolate can often normalize homocysteinemia.49
RBC Omega-3 Index
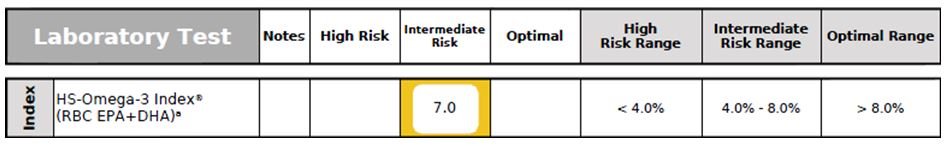
Figure 8. Omega-3 Index
Omega-3 Index (RBC EPA + DHA) not only provides a clinically reliable evaluation of omega-3 fatty acid status, but is also a CV risk factor. A low Omega-3 Index has been associated with sudden cardiac death, acute coronary syndromes, accelerated cellular aging, early mortality, impaired cognitive function, and increased risk for developing AD.50-53 This patient’s Omega-3 Index status was suboptimal (Figure 8).
Iron Panel
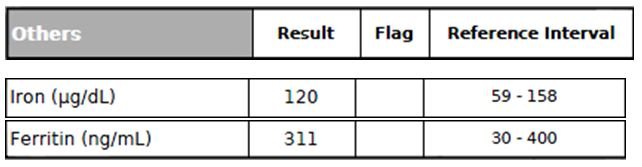
Figure 9. Iron Panel
Excessive accumulation of iron in the body (iron overload) is a strong risk factor for a wide range of diseases, including CVD, liver dysfunction, peripheral arterial disease, and neurodegenerative diseases, to name a few.54 Researchers have also found that hyperferritinemia can be linked to insulin resistance and fatty liver in the absence of iron overload (determined by liver biopsies).54 In this case (Figure 9), the patient had a normal serum Iron level but a borderline-high Ferritin level (which could be due to insulin resistance and fatty liver, especially considering his borderline-high ALT level).
Comprehensive Diabetes Panel
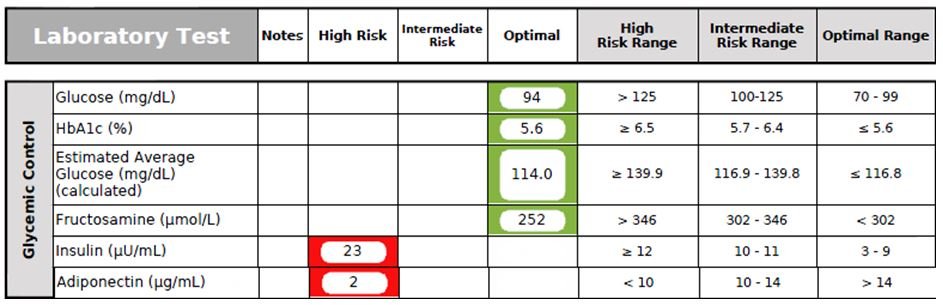
Figure 10. Diabetes Panel
- Fasting Glucose (See CMP section)
- Hemoglobin A1c (HbA1c), a well-established diabetic screening test that provides a picture of the average amount of blood glucose for the past 2-3 months, unlike a fasting blood glucose, which only provides a snapshot. HbA1c is also a good test for monitoring compliance of dietary recommendations in patients who have prediabetes/diabetes. However, its level can be affected by several medical conditions, including anemia (iron and/or B12 deficiency), kidney or liver disease, recent severe bleeding or blood transfusion, and hemoglobin disorders. This patient’s HbA1C was borderline-high at 5.6% (Figure 10).
- Fructosamine (Glycated Serum Protein) provides an average blood sugar measurement for the past 2-3 weeks. Fructosamine is formed by non-enzymatic glycation of multiple serum proteins, including hemoglobin and albumin.55 Moreover, Fructosamine is not affected by RBC turnover or hemoglobinopathies, so can be used in specific clinical conditions that exclude the use of HbA1c.55 However, Fructosamine, because it is dependent on glycation of protein, will not be a reliable marker in conditions in which global protein metabolism is altered, such as nephrotic syndrome and liver cirrhosis. The patient’s Fructosamine level was found to be normal.
- Adiponectin has been shown to be a crucial molecule involved in limiting the pathogenesis of obesity-linked disorders, and may have potential benefits in the treatment and prevention of CVD. High levels of adiponectin are linked to a reduced risk of CAD and increased endothelial nitric oxide production.56 Hypoadiponectinemia is associated with the pathogenesis of type 2 diabetes, CAD, hypertension, and left ventricular hypertrophy.56 In this case, the patient’s adiponectin level was very low.
- Fasting Insulin, especially when combined with elevated Triglycerides and a high waist-to-hip ratio, provides an excellent estimate of current insulin resistance status and risk of future CVD.57 Insulin is responsible for regulating blood glucose by facilitating uptake of glucose by cells for energy or for storage. Progressively elevated fasting insulin levels is significantly associated with insulin resistance as well as atherosclerosis and CVD risk. This patient’s fasting insulin level was highly elevated at 23 mU/mL, indicating significant insulin resistance and MetSyn.
Comprehensive Thyroid Panel
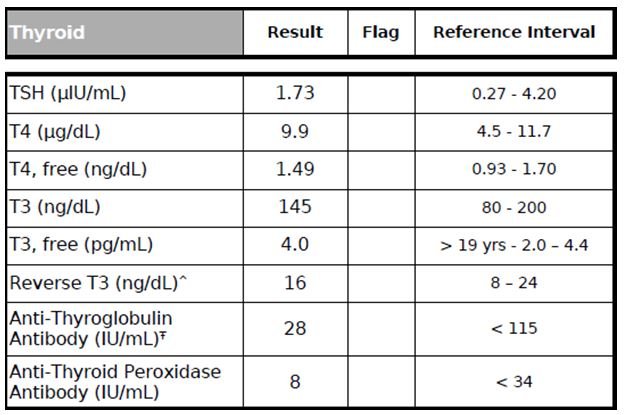
Figure 11. Thyroid Panel
Thyroid hormones can have a huge impact on the CV system.58-60 For example, hyperthyroidism has been linked to tachycardia, atrial arrhythmias, increased left ventricular mass, impaired ventricular relaxation, poor exercise performance, and increased risk of cardiovascular mortality.58-60 On the other hand, hypothyroidism has been linked with impaired left ventricular diastolic function, systolic dysfunction, and an increased risk of atherosclerosis and MI.58-60 Therefore, it is essential to get a complete assessment of thyroid function with the following tests: TSH, free T3, total T3, free T4, total T4, Reverse T3, and Thyroid Antibodies (Anti-TPO & Anti-Thyroglobulin Antibodies). In this case, the patient has normal thyroid parameters (Figure 11).
Sex Hormones
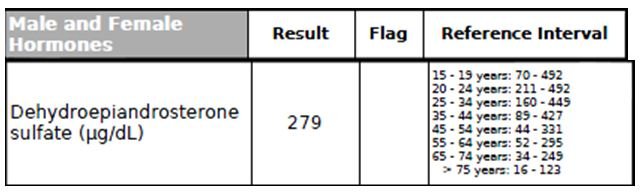
Figure 12. DHEA
The adrenal sex hormone, dehydroepiandrosterone (DHEA), mainly present in the body as sulfate ester (DHEA-S), is the most abundant steroid hormone in human blood. DHEA has been shown to be crucial for body composition, insulin sensitivity, endothelial function, female fertility metabolism, and neuronal/CNS functions.61,62 Moreover, DHEA is considered to have a cardioprotective effect by reducing vascular inflammation and remodeling, platelet aggregation, oxidative stress, and atherosclerosis.61 Low serum levels of DHEA-S have also been linked to age-related cardiometabolic diseases and an increased risk of coronary events.61 This patient’s DHEA-S level was normal (Figure 12).
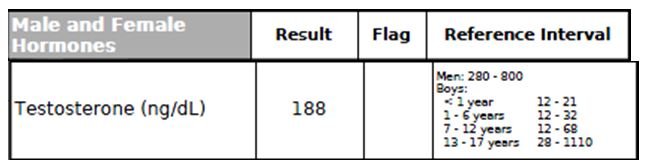
Figure 13. Testosterone
Several longitudinal population studies have demonstrated that testosterone deficiency is associated with an increase in all-cause mortality, including cardiovascular, respiratory, and cancer deaths.63 Testosterone deficiency is linked to several key CV risk factors, including central obesity, insulin resistance, hyperglycemia, dyslipidemia, chronic inflammation, and hypertension.63 This patient’s Testosterone level was found to be low (Figure 13).
Summary
In summary, the comprehensive cardiometabolic assessment revealed that this patient had been suffering from metabolic syndrome (obesity, high Triglycerides, low HDL-C, and insulin resistance with hyperinsulinemia and reduced Adiponectin), increased inflammatory markers (elevations in Fibrinogen, hs-CRP, Uric acid, Hcy, and MPO), vitamin D deficiency, borderline B12 and folate deficiencies, elevated liver enzymes (ALT and GGT), and low Testosterone. Part 2 of this article will discuss the comprehensive naturopathic treatment principles and plan used to address these abnormalities, as well as the follow-up lab results following his treatment.
References:
- Centers for Disease Control and Prevention. Heart Disease Facts. Last updated August 10, 2015. CDC Web site. http://www.cdc.gov/heartdisease/facts.htm. Accessed July 1, 2016.
- The Heart Foundation. Heart Disease: Scope and Impact. The Heart Foundation Web site. http://www.theheartfoundation.org/heart-disease-facts/heart-disease-statistics/. Accessed July 1, 2016.
- International Diabetes Federation. IDF Worldwide Definition of The Metabolic Syndrome. IDF Web site. http://www.idf.org/metabolic-syndrome. Accessed July 1, 2016.
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. IDF Web site. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed July 1, 2016.
- Grundy SM, Brewer HB Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433-438.
- Takamiya T, Zaky WR, Edmundowics D, et al. World Health Organization-defined metabolic syndrome is a better predictor of coronary calcium than the adult treatment panel III criteria in American men aged 40-49 years. Diabetes Care. 2004;27(12):2977-2979.
- Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48.
- Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029.
- Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111-117.e2.
- Bjørnholt JV, Erikssen G, Aaser E, et al. Fasting blood glucose: an underestimated risk factor for cardiovascular death. Results from a 22-year follow-up of healthy nondiabetic men. Diabetes Care. 1999;22(1):45-49.
- Chen S, Guo X, Yu S, et al. Metabolic Syndrome and Serum Liver Enzymes in the General Chinese Population. Int J Environ Res Public Health. 2016;13(2):223.
- Kim WR, Flamm SL, Di Bisceglie AM, et al. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363-1370.
- Onur S, Niklowitz P, Jacobs G, et al. Ubiquinol reduces gamma glutamyltransferase as a marker of oxidative stress in humans. BMC Res Notes. 2014;7:427
- Framingham Heart Study. Cardiovascular Disease (10-year risk). Framingham Heart Study Web site. https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php#. Accessed July 1, 2016.
- Miller M, Stone, MJ, Ballantyne C, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292-2333.
- Gaziano JM, Hennekens CH, O’Donnell CJ, et al. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation.1997;96(8):2520-2252.
- Nesto RW. Beyond low-density lipoprotein: addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am J Cardiovasc Drugs. 2005;5(6):379-387.
- Musunuru K, Orho-Melander M, Caulfield MP, et al. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29(11):1975-1980.
- Ai M, Otokozawa S, Asztalos BF, et al. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56(6):967-976.
- Meeusen JW, Donato LJ, Jaffe AS. Should apolipoprotein B replace LDL cholesterol as therapeutic targets are lowered? Curr Opin Lipidol. 2016;27(4):359-366.
- Oravec S, Dostal E, Dukát A, et al. HDL subfractions analysis: a new laboratory diagnostic assay for patients with cardiovascular diseases and dyslipoproteinemia. Neuro Endocrinol Lett. 2011;32(4):502-509.
- Mangaraj M, Nanda R, Panda S. Apolipoprotein A-I: A Molecule of Diverse Function. Indian J Clin Biochem. 2016;31(3):253-259.
- Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128(11):1189-1197.
- Capoulade R, Chan KL, Yeang C, et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J Am Coll Cardiol. 2015;66(11):1236-1246.
- Qi Q, Workalemahu T, Zhang C, et al. Genetic variants, plasma lipoprotein(a) levels, and risk of cardiovascular morbidity and mortality among two prospective cohorts of type 2 diabetes. Eur Heart J. 2012;33(3):325-334.
- Tselepis AD. Oxidized phospholipids and lipoprotein-associated phospholipase A(2) as important determinants of Lp(a) functionality and pathophysiological role. J Biomed Res. 2016 Apr 2;31. [Epub ahead of print]
- Corban MT, Hung OY, Mekonnen G, et al. Elevated Levels of Serum Fibrin and Fibrinogen Degradation Products Are Independent Predictors of Larger Coronary Plaques and Greater Plaque Necrotic Core. Circ J. 2016;80(4):931-937.
- Corrado E, Novo S. Role of inflammation and infection in vascular disease. Acta Chir Belg. 2005;105(6):567-579.
- Tabakcı MM, Gerin F, Sunbul M, et al. Relation of Plasma Fibrinogen Level With the Presence, Severity, and Complexity of Coronary Artery Disease. Clin Appl Thromb Hemost. 2016 Feb 9. [Epub ahead of print]
- Lachine NA, Elnekiedy AA, Megallaa MH, et al. Serum chemerin and high-sensitivity C reactive protein as markers of subclinical atherosclerosis in Egyptian patients with type 2 diabetes. Ther Adv Endocrinol Metab. 2016;7(2):47-56.
- Maiolino G, Bisogni V, Rossitto G, Rossi GP. Lipoprotein-associated phospholipase A2 prognostic role in atherosclerotic complications. World J Cardiol. 2015;7(10):609-620.
- Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343(16):1148-1155.
- Michowitz Y, Kisil S, Guzner-Gur H, et al. Usefulness of serum myeloperoxidase in prediction of mortality in patients with severe heart failure. Isr Med Assoc J. 2008;10(12):884-888.
- Duivenvoorden R, Mani V, Woodward M, et al. Relationship of serum inflammatory biomarkers with plaque inflammation assessed by FDG PET/CT: the dal-PLAQUE study. JACC Cardiovasc Imaging. 2013;6(10):1087-1094.
- Ilisson J, Zagura M, Zilmer K, et al. Increased carotid artery intima-media thickness and myeloperoxidase level in children with newly diagnosed juvenile idiopathic arthritis. Arthritis Res Ther. 2015;17:180.
- Huang Y, Wu Z, Riwanto M, et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J Clin Invest. 2013;123(9):3815-3828.
- Wu J, Qiu L, Guo XZ, et al. Apolipoprotein E gene polymorphisms are associated with primary hyperuricemia in a Chinese population. PLoS One. 2014;9(10):e110864.
- Erol M, Gayret OB, Yigit O, et al. A Case of Homocystinuria Misdiagnosed as Moyamoya Disease: A Case Report. Iran Red Crescent Med J. 2016;18(4):e30332.
- Li WX, Dai SX, Zheng JJ, et al. Homocysteine Metabolism Gene Polymorphisms (MTHFR C677T, MTHFR A1298C, MTR A2756G and MTRR A66G) Jointly Elevate the Risk of Folate Deficiency. Nutrients. 2015;7(8):6670-6687.
- Kotze MJ, van Rensburg SJ. Pathology supported genetic testing and treatment of cardiovascular disease in middle age for prevention of Alzheimer’s disease. Metab Brain Dis. 2012;27(3):255-266.
- Vučinić N, Djan I, Stokić E, et al. Different associations of apoE gene polymorphism with metabolic syndrome in the Vojvodina Province (Serbia). Mol Biol Rep. 2014;41(8):5221-5227.
- Teixeira AA, Marrocos MS, Quinto BM, et al. Diversity of apolipoprotein E genetic polymorphism significance on cardiovascular risk is determined by the presence of metabolic syndrome among hypertensive patients. Lipids Health Dis. 2014;13:174.
- Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl). 2016;94(7):739-746.
- Raj R, Bhatti JS, Badada SK, Ramteke PW. Genetic basis of dyslipidemia in disease precipitation of coronary artery disease (CAD) associated type 2 diabetes mellitus (T2DM). Diabetes Metab Res Rev. 2015;31(7):663-671.
- Wierzbicka J, Piotrowska A, Żmijewski MA. The renaissance of vitamin D. Acta Biochim Pol. 2014;61(4):679-686.
- Khaw KT, Luben R, Wareham N. Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: a 13-y prospective population study. Am J Clin Nutr. 2014;100(5):1361-1370.
- Davì G, Santilli F, Patrono C. Nutraceuticals in diabetes and metabolic syndrome. Cardiovasc Ther. 2010;28(4):216-226.
- Bian S, Gao Y, Zhang M, et al. Dietary nutrient intake and metabolic syndrome risk in Chinese adults: a case-control study. Nutr J. 2013;12:106.
- Ambrosino P, Lupoli R, Di Minno A, et al. Cyclic supplementation of 5-MTHF is effective for the correction of hyperhomocysteinemia. Nutr Res. 2015;35(6):489-495.
- Tan ZS, Harris WS, Beiser AS, et al. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78(9):658-664.
- Roth EM, Harris WS. Fish oil for primary and secondary prevention of coronary heart disease. Curr Atheroscler Rep. 2010;12(1):66-72.
- Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26(6):988-995.
- Shearer GC, Pottala JV, Spertus JA, Harris WS. Red blood cell fatty acid patterns and acute coronary syndrome. PLoS One. 2009;4(5):e5444.
- Felipe A, Guadalupe E, Druso P, et al. Serum Ferritin Is Associated with Metabolic Syndrome and Red Meat Consumption. Oxid Med Cell Longev. 2015;2015:769739.
- Zheng CM, Ma WY, Wu CC, Lu KC. Glycated albumin in diabetic patients with chronic kidney disease. Clin Chim Acta. 2012;413(19-20):1555-1561.
- Lopez-Jaramillo P. The Role of Adiponectin in Cardiometabolic Diseases: Effects of Nutritional Interventions. J Nutr. 2016;146(2):422S-426S.
- Venkataraman K, Khoo CM, Leow MK, et al. New measure of insulin sensitivity predicts cardiovascular disease better than HOMA estimated insulin resistance. PLoS One. 2013;8(9):e74410.
- Vargas-Uricoechea H, Sierra-Torres CH. Thyroid hormones and the heart. Horm Mol Biol Clin Investig. 2014;18(1):15-26.
- Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31-50.
- Wu J, Tao Y, Gu H, Sui J. Association Between Cardiovascular Risk Factors and Serum Thyrotropin Concentration Among Healthy Chinese Subjects and Subjects with Unsuspected Subclinical Hypothyroidism. Clin Lab. 2016;62(5):807-814.
- Tivesten Å, Vandenput L, Carlzon D, et al. Dehydroepiandrosterone and its sulfate predict the 5-year risk of coronary heart disease events in elderly men. J Am Coll Cardiol. 2014;64(17):1801-1810.
- Prough RA, Clark BJ, Klinge CM. Novel mechanisms for DHEA action. J Mol Endocrinol. 2016;56(3):R139-R155.
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725-733.
Image Copyright: <a href=’https://www.123rf.com/profile_guniita’>guniita / 123RF Stock Photo</a>
 Teerawong Kasiolarn, ND, MSAc, LAc, received his Doctorate of Naturopathic Medicine and Master of Science in acupuncture from Bastyr University in 2005. After graduation, he served as a naturopathic resident at UBCNM in CT, Yale-Prevention Research Center, and the Integrative Medicine Center at Griffin Hospital, a teaching facility affiliated with Yale University. For the 10 years since his residency, Dr Kasiolarn has been practicing at Nova Medical Group, the largest integrative primary care practice in Northern VA, as a naturopathic doctor and licensed acupuncturist. Dr Kasiolarn focuses on CVD, metabolic syndrome, diabetes, thyroid disorders, weight management/obesity, chronic pain, chronic fatigue, digestive disorders, anxiety/depression, and autoimmune diseases.
Teerawong Kasiolarn, ND, MSAc, LAc, received his Doctorate of Naturopathic Medicine and Master of Science in acupuncture from Bastyr University in 2005. After graduation, he served as a naturopathic resident at UBCNM in CT, Yale-Prevention Research Center, and the Integrative Medicine Center at Griffin Hospital, a teaching facility affiliated with Yale University. For the 10 years since his residency, Dr Kasiolarn has been practicing at Nova Medical Group, the largest integrative primary care practice in Northern VA, as a naturopathic doctor and licensed acupuncturist. Dr Kasiolarn focuses on CVD, metabolic syndrome, diabetes, thyroid disorders, weight management/obesity, chronic pain, chronic fatigue, digestive disorders, anxiety/depression, and autoimmune diseases.