Intracrine Steroid Biosynthesis
ANDREW L. RUBMAN, ND, FABNE
SUSAN GORDON, PHD, LMT
In the May 2021 issue of NDNR, Carrie Decker, ND, wrote an excellent article titled “The Anti-Aging Effects of DHEA.”1 The present article also discusses DHEA, but focuses specifically on the evolutionary significance and clinical implications of the intracrine biosynthesis and metabolism of dehydroepiandrosterone (DHEA) in postmenopausal women. Forty percent of androgens in men, and 75% and nearly 100% of estrogens in women before and after menopause, respectively, are synthesized intracrinologically in peripheral target tissues from adrenal precursor steroids.2 This unique mechanism of androgen precursor activation, action, and inactivation in peripheral tissues is associated with classical genomic androgen signaling.3 DHEA is used primarily as a general anti-aging therapy and treatment for sexual dysfunction and cognitive/mood symptoms in peri- and postmenopausal women. Significant progress has also been made in understanding the tissue specificity of DHEA associated with hormone-dependent cancers and metabolic dysregulation.
Endocrine vs Intracrine
Endocrine activity was formerly thought to involve only the following sequential steps: First, allosteric hormones are secreted from specialized glands and transported to target cells. These hormones then bind to cell surface receptors on the target cells, causing hormones from one cell to either influence neighboring cells (paracrine) or exert a positive or negative action on the same cell (autocrine).2 The rate of biosynthesis is regulated by a homeostatic negative-feedback control mechanism: a hormone binds to a receptor that activates a signal transduction pathway that in turn activates gene transcription and expression of target proteins.
However, it is now recognized that hormones also act in rapid, non-genomic pathways that can be synergistic with genomic effects. Water-soluble hormones (peptides and amines) act on the surface of target cells via second messengers, and lipid-soluble hormones (steroids) pass through the plasma membranes of target cells (nuclear and cytoplasmic) to act within the cells’ nuclei. Simply stated, intracrine modulation of cellular function relies instead on intracellular processing of an endogenously synthesized compound, followed by a signal transduction pathway of the finally processed molecule, all within the same cell.4
The term intracrine refers to peptide/protein hormones that have intracellular actions, acting within the cell that synthesizes them, and exerting their action within this same cell, without exit and reentry. It is believed that the link between hormone receptors and unsuitable ligands is an intracellular enzyme that converts an inactive prohormone into a ligand that can activate that receptor and generate a biological response.4 Using DHEA as an example, Labrie et al, in their animal research 33 years ago, showed that the inactive form of this adrenocortical precursor from gonads, adrenals, and the brain could be imported into the cell and metabolized intracellularly to its downstream active sexual and adrenal steroids.5
Intracrinology, as developed by Labrie et al, thus studies the intracellular synthesis and metabolism of hormones in peripheral tissues, or aggregates of cells with similar structure and function occurring in the same cell in which synthesis (or inactivation) of sex steroids takes place, without significant release into the extracellular space, pericellular compartment, or general circulation.2,5 Labrie argued that the “inactive” adrenal steroid, DHEA, is the most important precursor of bioactive androgens in women after the cessation of gonadal biosynthesis in menopause. Peripheral tissue metabolism is a way for tissues to respond to local physiological demands (in immediately stressful situations) and “fine-tune” the activation or inhibition of steroid-hormone-receptor-dependent processes while also avoiding systemic exposure to high levels of circulating hormones such as testosterone and estrogen.6,7 This has important evolutionary and clinical significance, as discussed further below.
Metabolism of DHEA & DHEAS
Adrenal production of C19 androgen steroids (most notably DHEA), initiated during adrenarche with the maturation of the zona reticularis in the adrenal glands (approximately age 8 in girls and age 9 in boys8), is unique to humans and higher primates9 and represents the onset of intracrine androgen signaling in human life. A peak in adrenal C19 levels is reached in the third and fourth decades of life in both men and women, after which levels significantly decline.10 As mentioned above, close to half of all androgens in men and the majority of estrogens in women are synthesized intracrinologically in peripheral target tissues from adrenal precursors. This distinctive mechanism of androgen precursor activation, action, and inactivation in peripheral target cells is linked to classical genomic androgen signaling in both men and women.2,5,7,10,11 For interested readers, Labrie and others have written extensively on the intracrine metabolism of DHEA.10,12-19
DHEA and its sulfated ester, DHEAS, constitute the most abundant steroid hormones in the body.20 DHEAS is the stable, physiologically inactive, systemic form of DHEA in circulation, is synthesized de novo from LDL-cholesterol in the cytoplasm of most tissues, and serves as a reserve for the active form of the hormone. DHEAS is rendered active when the steroid sulfatase (STS) enzyme, expressed in all human tissues, catalyzes the hydrolysis of sulfate esters to produce DHEA. STS can be regarded as a fine-tuning mechanism for controlling levels of intracellular free steroids.21
Pregnenolone, synthesized from LDL-cholesterol within the cytoplasm, converts to 17-hydroxy-pregnenolone, DHEA, estrogen, and testosterone, as well as progesterone and the adrenocortical metabolites including the glucocorticoids (cortisol, cortisone, aldosterone) and the mineralocorticoid, aldosterone (see Figure 1). The glucocorticoids regulate glucose metabolism and control inflammation, while aldosterone regulates blood pressure and fluid balance via its action on electrolytes.22
Figure 1. Biosynthesis of Selected Steroids22
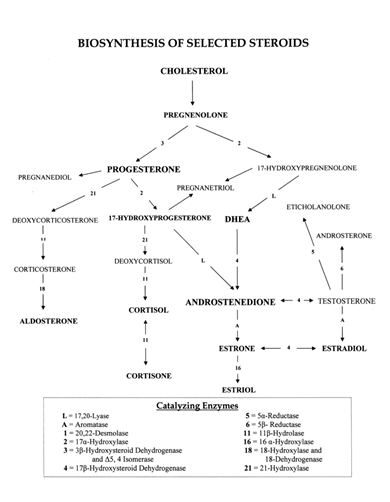
In intracrine steroid metabolism, DHEA is enzymatically activated to local androgens that then exert their effects via the androgen receptor. These active androgens are subsequently enzymatically inactivated before being excreted from the cell.3 Only in humans and the great apes can active steroids from DHEA be inactivated, processed within the cytoplasm, and removed from the cytoplasm into the general circulation.4
DHEA/DHEAS, is primarily synthesized locally, de novo in the brain, and transported across the blood-brain barrier.23-25 In the brain, DHEA/DHEAS, as neurosteroids, modulate: the GABA-A receptor (inhibitory, acting as noncompetitive antagonists), the NMDA receptor (positive allosteric modulator), and the sigma subtype 1 receptor (regulates ion channel activity, neurotransmitter release, induces extracellular acetylcholine, and promotes calcium transport through NMDA). DHEA is also believed to interact with cytoskeleton components, novel membrane receptors, and receptor sites in the peripheral and central nervous systems.3 Additionally, DHEA modulates a variety of cholinergic, dopaminergic, and glutamatergic synaptic transmissions in which circulating bioavailable androgens and their precursors cross the plasma membrane of target cells to be metabolized by intracellular enzymes in the endoplasmic reticulum or the cytosol.3 While unconjugated steroids can diffuse across the membrane due to their hydrophobic nature, steroid conjugates (sulfates and glucuronides) are hydrophilic and require active transport.26
Clinical Implications
The many symptoms and medical problems experienced by postmenopausal women can have a negative impact on their quality of life and even their lifespan. These problems can include hot flushes, night sweats, vulvovaginal atrophy, muscle loss, bone loss and fractures, sexual dysfunction, and cognitive impairments, including memory loss and Alzheimer’s disease. The severity of the symptomatology related to hormone deficiency varies between races and from person to person. With longer lifespans and a growing world population, more than 1.1 billion women worldwide are expected to be postmenopausal by the year 2025.27 Restoring hormonal functions without increasing disease risks associated with excessive hormone levels is ideal. Intracrine effects make this more feasible in that intracellularly produced estrogens and androgens can exert their effects but then be inactivated before exiting the cell.7
Since adrenal-derived DHEA becomes the unique source of sex steroids in women after menopause and these sex steroids are produced intracellularly, serum estrogen and testosterone are maintained at low biological concentrations, with no activity outside the cells of origin. It is therefore clinically safe and efficacious to replace DHEA in women suffering from vulvovaginal atrophy by using oral sublingual drops, topical cream, or intravaginal administration.7
Evolution has led to menopause being a convergence of the cessation of ovarian function, high circulating DHEA, and intracrine enzymes that can convert DHEA into active sex hormones in peripheral tissues. Although the steep decline in ovarian estrogen production at menopause protects the endometrium from cancer, ongoing availability of sex steroids to other tissues is important for maintaining normal functioning. Intracellular formation of estrogens and androgens from DHEA makes this possible while also preventing clinically significant release of sex steroids into the general circulation. Thus, DHEA supplementation serves an evolutionary, protective, and health-promoting function.
Because intracellular steroids are inactivated by sulfatase and glucuronyl transferase enzymes before leaving the cell, blood measurement of circulating sex steroids provides limited information about hormone activity and concentrations in peripheral tissues. Urine collection is a preferable matrix for steroid analysis, as it represents the total of all steroid biosynthetic and metabolizing pathways. However, to date, only a limited number of methods for the simultaneous measurement of free, sulfated, and glucuronidated androgens have been established. Technological advances using gas chromatography – tandem mass spectrometry (GC – MS/MS) have helped provide evidence of tissue-specific concentrations of steroids that do not parallel those in circulation28 and advanced our understanding of enzymatic pathways that are regulated intracrinologically.
References
- Decker C. The Anti-Aging Effects of DHEA. NDNR. 2021;17(5):14-16.
- Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78(3):C113-C118.
- Schiffer L, Arlt W, Storbeck, KH. Intracrine androgen biosynthesis, metabolism and action revisited. Mol Cell Endocrinol. 2018;465:4-26.
- Kühn-Velten WN. Intracrinology and the local enzymatic control of hormone distribution and metabolism: dehydroepiandrosterone does not just act as a prohormone for androgens and estrogens. Eur J Clin Invest. 2000;30 Suppl 3):34-38.
- Labrie C, Bélanger A, Labrie F. Androgenic activity of dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology. 1988;123(3):1412-1417.
- Labrie F. Intracrinology: the new science of sex steroid physiology in women. In: Birkhaeuser M, Genazzani AR, eds. Pre-menopause, Menopause and Beyond. ISGE Book Series. Cham, Switzerland: Springer; 2018:3-16.
- Labrie F, Bélanger A, Pelletier G, et al. Science of intracrinology in postmenopausal women. Menopause. 2017;24(6):702-712.
- Idkowiak J, Lavery GG, Dhir V, et al. Premature adrenarche: novel lessons from early onset androgen excess. Eur J Endocrinol. 2011;165(2):189-207.
- Arlt W, Martens JWM, Song M, et al. Molecular evolution of adrenarche: structural and functional analysis of P450c17 from four primate species. Endocrinology. 2002;143(12):4665-4672.
- Labrie F, V. Luu-The V, Labrie C, et al. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22(3):185-212.
- Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J Steroid Biochem Mol Biol. 2015;145:133-138.
- Burger HG. Androgen production in women. Fertil Steril. 2002;77 Suppl 4:S3-S5.
- Labrie F. Extragonadal synthesis of sex steroids: intracrinology. Ann Endocrinol (Paris). 2003;64(2):95-107.
- Labrie F, Belanger A, Cusan L, et al. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab. 1997;82(8):2403-2409.
- Labrie F, Bélanger A, Luu-The V, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1988;63(5-6):322-328.
- Labrie F, Belanger A, Simard, J, et al. Intracrinology: autonomy and freedom of peripheral tissues. Ann Endocrinol (Paris). 1995;56(1):23-29.
- Labrie F, Luu-The V, Bélanger A, et al. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187(2):169-196.
- Simpson ER. Aromatization of androgens in women: current concept and findings. Fertil Steril. 2002;77 Suppl 4:S6-S10.
- Neunzig J, Bernhardt R. Dehydroepiandrosterone sulfate (DHEAS) stimulates the first step in the biosynthesis of steroid hormones. PLoS One. 2014;9(2):e89727.
- Reed MJ, Purohit A, Woo LE, et al. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocrinol Rev. 2005;26(2):171-201.
- Gordon S. Psychoneurointracrinology: the embodied self. Biosynthesis of selected steroids. In: Gordon S, ed. Neurophenomenology and Its Application to Psychology. New York, NY: Springer; 2013:130.
- Weill-Engerer S, David JP, Sazdovitch V, et al. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clin Endocrinol Metab. 2002;87(11):5138-5143.
- Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150 Suppl:S125-S127.
- Webb SJ, Geoghegan TE, Prough RA, et al. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev. 2006;38(1-2):89-116.
- Giorgi EP, Stein WD. The transport of steroids into animal cells in culture. Endocrinology. 1981;108(2):688-697.
- Shifren JL, Gass ML; NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21(10):1038-1062.
- Gibson DA, Simitsidellis I, Collins F, Saunders PTK. Endometrial Intracrinology: Oestrogens, Androgens and Endometrial Disorders. Int J Mol Sci. 2018;19(10):3276.

Andrew L. Rubman, ND, FABNE, is a 1982 graduate of NUNM and Medical Director of the Southbury Clinic for Traditional Medicines, in Southbury, CT. He is a founder and lifetime member of the American Association of Naturopathic Physicians, a Member of the Board of Medical Examiners of the Naturopathic Endocrine Society, and a Fellow of the Endocrine Association of Naturopathic Physicians. Dr Rubman has been active in national television, radio, and press, and with organizations advocating the evolution of a responsible medical continuum among all licensed physicians with unrestricted practice for naturopathic physicians.

Susan Gordon, PhD, LMT is an academic psychologist, core adjunct professor in the Department of Psychology at NUNM, research director of the Southbury Clinic for Traditional Medicines, licensed massage therapist, and counselor. Dr Gordon has a doctorate in the History and Philosophy of Psychology; Mind/Body Medicine from Saybrook University, and she trained in naturopathic medicine at Bastyr University. Her research and published writings focus on psychoneurointracrinology and the neurophenomenology of well-being. She is editor/author of Neurophenomenology and Its Applications to Psychology (Springer, 2013) and author of the forthcoming book, Psychoneurointracrinology: The Mind-Brain Continuum (Springer, 2021).