Prebiotics & Metabolic Regulation: Benefits Beyond the Gut
Tolle Totum
Erick R. Cervantes, MPH, ND
Dale Pfost, PhD
Human beings are host to a diverse ecosystem known as the microbiome. Characteristic of our microbiome, among many other things, is its now well-known diversity within the individual and from person to person, as well as its vast array of elaborate influences on our physiology. Our scientific knowledge of this symbiotic ecosystem has grown tremendously, and its clinical significance and implications cannot be ignored. This paper highlights how some microbiota within our intestinal tract, in particular the large intestine, influences our health through its fermentation of prebiotic dietary fiber and polyphenols and the resulting metabolite-driven feedback mechanisms. In addition, this paper discusses the clinical implications of prebiotic (dietary fiber and polyphenols) supplementation for various metabolic syndromes in terms of its influence on satiety and blood glucose regulation. To illustrate, we present findings from 2 clinical studies examining the health effects of a prebiotic blend referred to as a Gastrointestinal Microbiome Modulator, or GIMM. Both studies were conducted under the direction of Frank Greenway, MD, of the Pennington Biomedical Research Center in Baton Rouge, LA, one of the nation’s leading centers for nutrition research.
In general, our Western lifestyle plays a detrimental role in our health and well-being. Characteristic of a Standard American Diet (SAD) and Western lifestyle is a decrease in physical exercise (sedentary lifestyle) as well as an increase in energy intake in the form of increased dietary sugars and processed foods.1 Such diets have a high glycemic index, are high in calories, and contribute to both high blood sugar excursions and weight gain. Daily consumption of this type of diet has led to an increased incidence of complex symptoms collectively categorized as metabolic syndrome. The key characteristics of metabolic syndrome are obesity, loss of glycemic control, dyslipidemia, and hypertension.1 The relationship of these conditions with gut health and our microbiota is explained here. It is also important to note that associated luminal dysbiosis due to poor dietary choices and other factors – such as food sensitivities/allergies, exposure to pesticides in our food supply, alcohol abuse, and antibiotics – plays a major role in gut permeability and impaired gut-barrier function.2 The consequences are a variety of other associated clinical conditions: food sensitivities and allergies, allergic rhinitis, inflammatory bowel disease, celiac disease, autoimmune hepatitis, prediabetes, type 2 diabetes mellitus, multiple sclerosis, systemic lupus erythematosus, depression, and autism.
Prebiotics, SCFAs & Health
Our gastrointestinal (GI) health is greatly dependent not only on the foods we eat, but also on the health of our gut microbiota. The human microbiome is the entirety of the environment of microorganisms living in and on our bodies.3 The majority of them symbiotically inhabit our GI tract.
Diets that are high in fruit and vegetables are richer in fiber than the SAD diet. On average, Western diets provide approximately 20-25 grams of dietary fiber per day, while high fruit and vegetable diets provide approximately 60 grams of dietary fiber.1 Intestinal bacteria, specifically those residing in the cecum and large intestine, produce short-chain fatty acids (SCFAs) – key signaling metabolites from non-digestible nutrients, specifically fiber and polyphenols, that pass out of the small intestine unaffected.1 The major types of fiber that pass through the gut are plant cell-wall polysaccharides, oligosaccharides, and resistant starches (eg, inulin and beta-glucan). The relationship between the health of our microbiota and high-fiber diets has been well established. Interestingly, the amount and type of fiber consumed has dramatic effects on the composition of the microbiota and consequently the type and amount of SCFAs produced.
The health of our GI tract and the health of our microbiota are thus directly correlated to our dietary intake of fiber. More specifically, daily intake of dietary prebiotics (polyphenols and non-digestible complex carbohydrates, aka fiber) directly affects the diversity of our microbiota, our metabolism, and therefore our health. Prebiotics cannot be digested by humans but are fermentable and serve as nutrients for the microbiota in the large intestine. The SCFAs (the products of fermentation) are used by the cecal and colonic epithelium cells for energy production, and they lower luminal pH, which inhibits the growth of pathogenic bacteria.
SCFAs also have far-reaching actions beyond the intestine. For example, SCFAs act as signaling molecules to G-protein receptors involved in satiety and glucose regulation, and they regulate the balance between fatty acid synthesis, fatty acid oxidation, and lipolysis. Specifically, fatty acid oxidation is activated by SCFAs in the liver and muscle tissue, while de novo synthesis and lipolysis are inhibited. This results in a reduction of free fatty acids in the plasma and a decrease in body weight.1
SCFAs inhibit fat storage in adipose tissue by suppressing insulin signaling in adipocytes, while promoting lipid and glucose metabolism in other tissues.1 Moreover, SCFAs trigger the release of gut hormones, peptide YY (PYY) and glucagon-like peptide-1 (GLP1). PYY is known as a satiety hormone, helping to promote the feeling of satisfaction and early cessation of eating. GLP1 triggers the release of insulin and decreases the secretion of glucagon by the pancreas for glucose regulation; it also slows gastric emptying.4-7
Study #1
Prebiotic Effects on GI Microbes & SCFAs
In a placebo-controlled, double-blind, randomized trial, 30 adults with a body mass index (BMI) >30 or a diagnosis of prediabetes were administered either a prebiotic formulation or placebo for 4 weeks. Stool and blood samples were collected at baseline and at 4 weeks, and markers for intestinal microbiome health and metabolic parameters were measured.
For the GI portion of the study, a GIMM, a strategic blend of 3 prebiotic nutrients, was studied for its ability to promote a healthy-functioning gut microbiome.8 Fecal assessments included aerobic and anaerobic bacteria and yeast/fungi (as determined by DNA analysis); SCFAs; markers for , and inflammation; and secretory IgA (sIgA) to assess immune function. This prebiotic blend was specifically designed to produce a greater yield of SFCAs in the large intestine by diverting biosynthetic pathways towards the SCFAs and shifting away from biosynthesis of methane and hydrogen sulfide, gases that have been associated with obesity,9,10 type 2 diabetes,11 irritable bowel syndrome and colitis.12,13 The blend included inulin, beta-glucan, and polyphenols, totaling 8.8 grams of fiber in powdered form. A blend of xanthan gum and cellulose was used for the placebo. Participants were instructed to mix the powder into 6 oz water and take it twice daily on an empty stomach. No changes were made to their daily meal routine.
Inulin is a fermentable prebiotic that expands the microbiota populations, leading to an increased production of SCFAs (Figure 1).14 With the growth achieved by inulin, the second ingredient – polyphenols extracted from blueberries – serves as a microbial substrate that is acetogenic, ie, uses hydrogen to produce acetate, a key SCFA (Figure 2).15,16 This can result in a competitive advantage for the acetogens and helps shift the microbiome activity from microbiota that would otherwise produce methane and hydrogen sulfide. The third ingredient, beta-glucan, protects the GI tract by triggering the release of sIgA and acting as a decoy substrate for microbiota to consume instead of digesting the mucosal lining.15,17-21
Figure 1. Production of SCFAs from Prebiotics
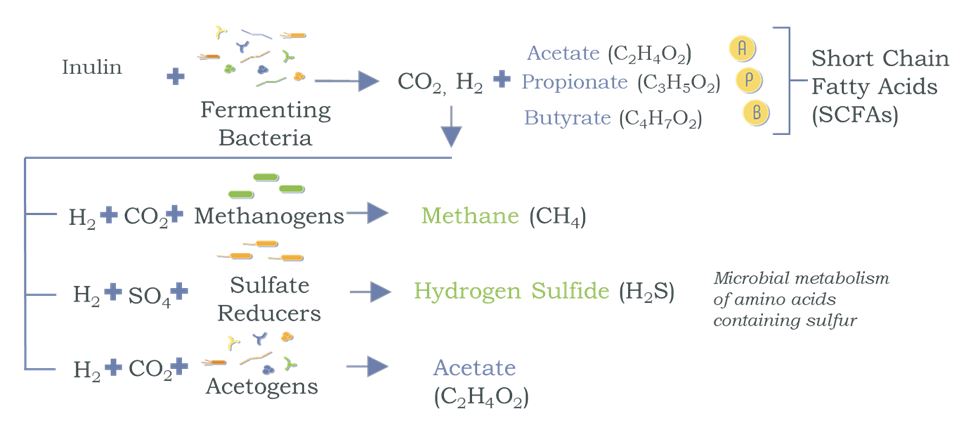
Cross-feeding of microbiota: Use of hydrogen from an initial fermentation produces undesirable gases and more SCFAs through a competitive process.
Figure 2. Polyphenols Shift the Competitive Balance of H2 Utilization
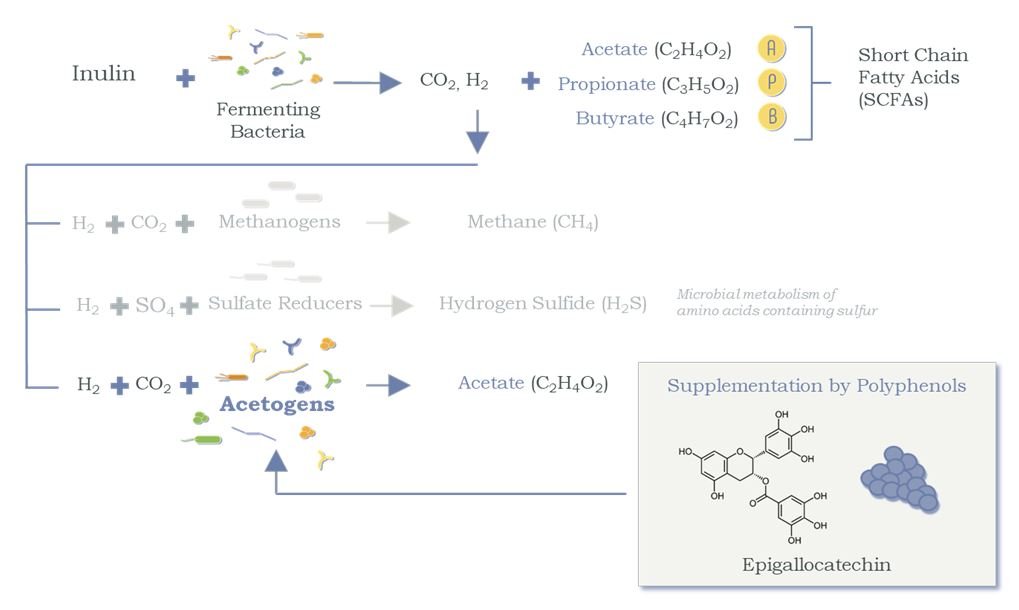
Polyphenols serve as a key substrate for acetogens to produce acetate (thus reducing biosynthesis of undesirable gases).
Summary of Prebiotic Blend
- Inulin from agave promotes growth of microbial populations that produce SCFAs
- Polyphenol antioxidants from blueberries provide a substrate for the growth and activity of certain microbiota that produce acetate, a key SCFA
- Beta-glucan from oats protects the intestine’s mucosal lining and supports the immune system
Prebiotic Effects on Glucose Regulation & Appetite
This same study8 also assessed the effectiveness of GIMM on metabolic parameters, lipids, and satiety, at baseline and at 4 weeks. Evidence supports the significant role of the gut microbiota in the biochemistry of satiety – the feeling of reduced hunger and desire for smaller portions at mealtime.22 A validated Visual Analog Scale (VAS) was utilized to assess satiety. Blood measurements included serum glucose and insulin (in a 3-hour oral glucose tolerance test [GTT]), hemoglobin A1c, hs-C-reactive protein (hsCRP), PYY, ghrelin, lipids, and other blood chemistry.
Compared to placebo, results for the prebiotic group showed reduced mealtime blood glucose measurements, as measured during the oral GTT (Figure 3); increased satiety, as measured by the VAS (Figure 4), and increased levels of sIgA, a fecal biomarker for immune preparedness (Figure 5).
Figure 3. Serum Glucose After 4 Weeks of Prebiotics
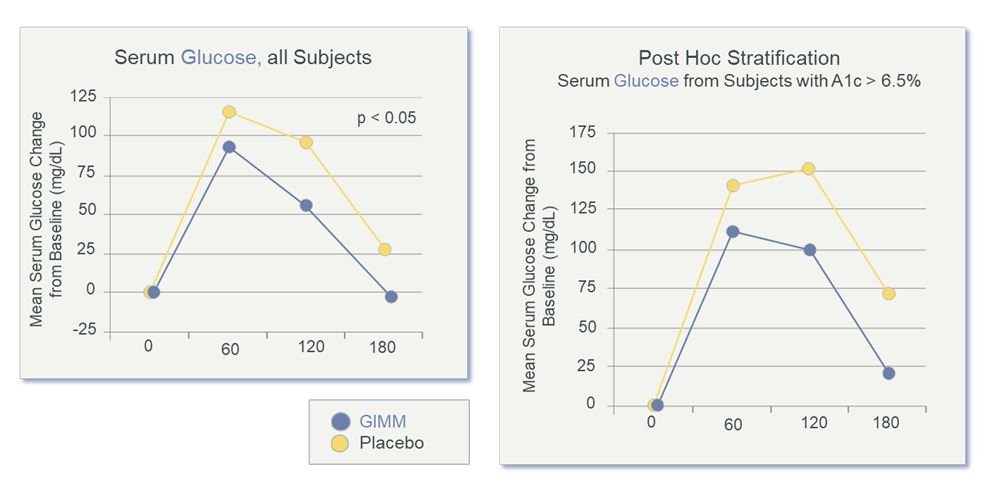
Note: Blood glucose excursion was significantly improved after 4 weeks. There was a significant postprandial effect and greater effect in diabetic subjects (those with A1c >6.5%). Fasting blood glucose normalized to 0. (GIMM = Gastrointestinal Microbiome Modulator)
Figure 4. Increased Satiety After 4 Weeks of Prebiotics
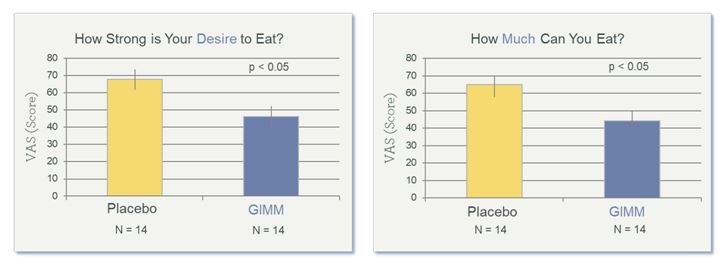
A Validated Visual Analog Scale (VAS) was used to measure appetite. Data shown are from the final visit (Week 4). (GIMM = Gastrointestinal Microbiome Modulator)
Figure 5. SIgA After 4 Weeks of Prebiotics
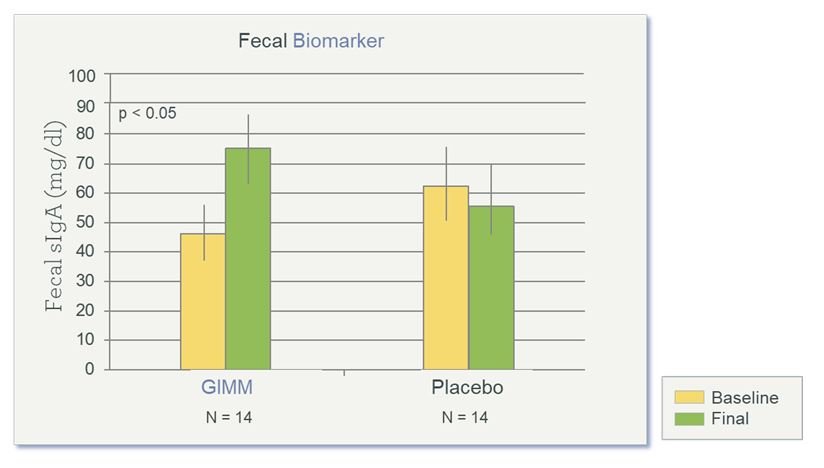
The intestinal antibody sIgA normally defends against food allergens and pathogens. Study showed significantly increased sIgA at the final visit (Week 4). (GIMM = Gastrointestinal Microbiome Modulator)
Study #2
Effect of Prebiotics on Metformin Tolerability
Not all drugs developed to be taken orally are completely absorbed into the blood and thus remain in the GI tract where there is potential for interactions with the microbiota. Individuals with high blood sugar are commonly offered metformin, an effective glucose-lowering therapeutic that is generally well tolerated; however, the drug can occasionally lead to diarrhea, constipation, heartburn, or nausea. For some people, this is enough to make it a challenge for them to achieve the recommended dosing. This second study showed that the same prebiotic blend (GIMM) provides nutritional support to counterbalance the occasional gastrointestinal effects that can accompany metformin.
This placebo-controlled, double-blind, randomized, crossover study assessed type 2 diabetics with documented metformin intolerance.23 Each of the 2 treatment periods lasted 2 weeks, with a 2-week washout period between them. All subjects continued to take daily metformin. In addition, subjects ingested either placebo (cellulose) or the GIMM. No changes were made to their daily meal routines. Frequency and consistency of bowel movements and metformin tolerability were assessed via questionnaires, and fasting blood glucose was monitored via morning finger-stick tests.
Results showed improved tolerance of metformin (Figure 6) in the prebiotic group, as compared to the placebo group, as well as improved bowel movement regularity and consistency (loose-to-firm stools), all without compromising the drug’s usual therapeutic effect on regulating glucose levels.
In summary, the 2 clinical studies discussed here demonstrate that this prebiotic blend was effective at reducing the desire to eat and in reducing the amount of food anticipated or considered sufficient at the next meal, improving regularity from occasional GI disturbances, lowering mealtime blood sugar levels, protecting and preparing the GI immune system, and improving metformin tolerability.
Figure 6. Metformin Tolerability After 4 Weeks of Prebiotics
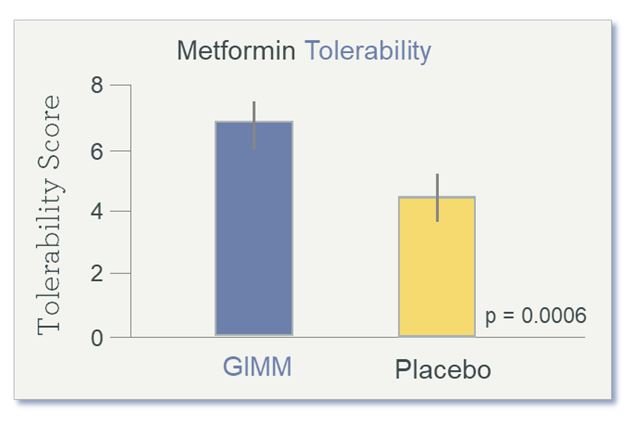
Tolerability Score is a symptom composite of bloating, urgency to evacuate, stool consistency, and flatulence. (GIMM = Gastrointestinal Microbiome Modulator)
Relevance of Prebiotics to SCFA Production
Dietary choices determine substrates for gut bacterial species, providing a competitive advantage to some microbes over others. As explained, dietary prebiotics (fiber and polyphenols) have a major role in the diversification of microbiota and those microbes’ subsequent role in host metabolism and overall health. The more diverse the diet, the more diverse the microbiota, and the more adaptable it can be to disruptions.
During the past 30 years, the prevalence of metabolic disorders has sharply increased24-26 while gut microbiome diversity has decreased.27,28 Additions or losses of species with similar roles tend to only have small effects on overall microbiome function. However, domination by a few species or a reduction in species diversity can significantly impact physiological functions, specifically those related to host metabolism and host defenses (ie, gut barrier function and immunity).
As evidenced by both human and animal studies, diet can cause a shift in microbial populations in as little as 3 days.29,30 Although temporary dietary changes will only briefly reduce diversity, some losses of microbiota may not be reversible after a prolonged absence of nutrients. This was suggested in a study in which mice inoculated with human microbiota and fed a low-fiber diet experienced an irreversible loss of microbial diversity over several generations despite a reintroduction of dietary fiber.31 In addition, human use of antibiotics, the use of antibiotics in meat production, and the use of pesticides in crop agricultural practices can narrow the microbiome.32-34 Pesticides can also subdue the plant’s own defense system, as the plant has no need to produce phytoalexins (a plant polyphenol with antimicrobial actions); the end result may be that key micronutrients that normally nourish the gut microbiome are no longer available when these plants are consumed.35
Such factors can jeopardize the microbial production of the SCFAs and thus affect host health. As the body of evidence grows supporting the role of SCFAs as key mediators of host metabolism, it becomes increasingly clear that SCFAs represent a key molecular link between diet, microbiota, and health.
Studies designed to improve microbial diversity using prebiotics have demonstrated some success, suggesting that high levels of non-digestible carbohydrates may be needed in Western diets to produce relevant changes in SCFA production, along with nutritional counseling and behavior modification surrounding dietary choices.
Fortunately, the increase in prevalence of type 2 diabetes, obesity, inactivity, and the ubiquitous SAD diet has led to an increasing focus on disease prevention. Prebiotics have been proven in numerous studies to alter the gut microbiome in favorable ways, and continuing research is now illustrating the power of prebiotics to impact health beyond the gut as well, especially metabolic disorders. SCFAs’ ability to affect appetite and glucose regulation suggests that they may play an important role in protecting the body against the deteriorating metabolic control and inflammation associated with Western lifestyles.
Nourishing the gut microbiota through diet and simple and cost-effective interventions such as prebiotics, including dietary fiber and polyphenols, offers significant promise in the prevention of metabolic syndrome and in slowing the progression of prediabetes to diabetes. As we all know, far more important than treating the symptom is to treat the cause.*
References:
- den Besten G, van Eunen K, Groen et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota and host energy metabolism. J Lipid Res. 2013;54(9):2325-2340.
- Meletis CD. The Leaky Gut/Allergy Catch-22: Underlying Trigger for Myriad Health Concerns. NDNR. 2018;14(8):1,4-5.
- Robinson CJ, Bohannan BJ, Young VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev. 2010;74(3):453-476.
- Utzschneider KM Kratz M, Damman CJ, Hullar M. Mechanisms Linking the Gut Microbiome and Glucose Metabolism. J Clin Endocrinol Metab. 2016;101(4):1445-1454.
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62(1):67-72.
- Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577-591.
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189-200.
- Rebello C, Burton J, Heiman M, Greenway FL. Gastrointestinal microbiome modulator improves glucose tolerance in overweight and obese subjects: A randomized controlled pilot trial. J Diabetes Complications. 2015;29(8):1272-1276.
- Yassour M, Lim MY, Yun HS, et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016;8(1):17.
- Barlow GM, Yu A, Mathur R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr Clin Pract. 2015;30(6):787-797.
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55-60.
- Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79.
- Singh SB, Lin HC. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms. 2015;3(4):866-889.
- Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35(1):9-16.
- Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57(1):1-24.
- Stull AJ, Cash KC, Johnson WD, et al. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140(10):1764-1768.
- El Khoury D, Cuda C, Luhovyy BL, Anderson GH. Beta glucan: health benefits in obesity and metabolic syndrome. J Nutr Metab. 2012;2012:851362.
- Wood PJ. Cereal β-glucans in diet and health. J Cereal Sci. 2007;46(3):230-238.
- Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323-335.
- Chan GC, Chan WK, Sze DM. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25.
- Cassidy YM, McSorley EM, Allsopp PJ. Effect of soluble fiber on postprandial glucose response and its potential as a functional food. J Funct Foods. 2018;46:423-439.
- De Silva A, Bloom SR. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver. 2012;6(1):10-20.
- Burton JH, Johnson M, Johnson J, et al. Addition of a Gastrointestinal Microbiome Modulator to Metformin Improves Metformin Tolerance and Fasting Glucose Levels. J Diabetes Sci Technol. 2015;9(4):808-814.
- Fryar CD, Carroll MD, Ogden CL, Division of Health and Nutrition Examination Surveys. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, 1960-1962 Through 2011-2012. July 2016. National Center for Health Statistics: Health E-Stats. CDC Website. https://www.cdc.gov/nchs/data/hestat/obesity_adult_13_14/obesity_adult_13_14.pdf. Accessed September 1, 2018.
- Olokoba AB, Obateru O, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269-273.
- Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12(38):6102-6108.
- Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085
- Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480-484.
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the humans gut microbiome. Nature. 2014;505(7484):559-563.
- Carmody RN, Gerber GK, Luevano JM, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72-84.
- Sonnenburg ED, Smits SA, Tikhonov M, et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212-215.
- Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003.
- Samsel A, Seneff S. Glyphosate’s Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases. Entropy. 2013;15(4):1416-1463.
- Gao B, Bian X, Mahbub R, Lu K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ Health Perspect. 2017;125(2):198-206.
- Hammerschmidt R. Phytoalexins: what have we learned after 60 years? Annu Rev Phytopathol. 1999;37:285-306.
* The statements in this paper have not been evaluated by the FDA. No product is implied or made reference to in this paper and all study materials or related supplements are not intended to diagnose, treat, cure, or prevent any disease.
Financial Disclosures: Dale Pfost is the founding CEO of MicroBiome Therapeutics, the company that provided the prebiotic blend used in the 2 studies discussed in this article. Dr Erick Cervantes is an ambassador for MicroBiome Therapeutics, and recommends a proprietary version of the GIMM to appropriate patients.
Acknowledgments: The authors wish to acknowledge the primary contribution to the research and development of the GIMM described here by Mark Heiman, PhD, who is the CSO emeritus of MicroBiome Therapeutics. Dr Heiman and Dr Frank Greenway are both scientific advisors to MicroBiome Therapeutics.
 Erick R. Cervantes, MPH, ND, is a 2011 graduate of NCNM in Portland, OR. Dr Cervantes is currently Program Chair and Assistant Professor of the Complementary and Alternative Health undergraduate program at Ashford University in San Diego, CA. He has taught within and led the program and has been practicing naturopathic medicine since 2011. Dr Cervantes joined the integrative health center, Body Craft, in October 2017. He uses an array of successful treatment interventions that include: Western herbs, homeopathy, massage, behavioral modification, nutrition, German bio-therapeutic drainage therapies, homeopathy, tissue cell salts, herbal stem-cell therapies, and micro-current therapy.
Erick R. Cervantes, MPH, ND, is a 2011 graduate of NCNM in Portland, OR. Dr Cervantes is currently Program Chair and Assistant Professor of the Complementary and Alternative Health undergraduate program at Ashford University in San Diego, CA. He has taught within and led the program and has been practicing naturopathic medicine since 2011. Dr Cervantes joined the integrative health center, Body Craft, in October 2017. He uses an array of successful treatment interventions that include: Western herbs, homeopathy, massage, behavioral modification, nutrition, German bio-therapeutic drainage therapies, homeopathy, tissue cell salts, herbal stem-cell therapies, and micro-current therapy.
***
 Dale Pfost, PhD, has 25 years of experience as a serial entrepreneur and executive in life sciences. He has been the founding CEO of 5 biotechnology companies – achieving 3 IPOs, 3 trade-sales and 3 companies with market capitalizations of over $2 billion. These include: Oxford GlycoSciences (IPO 1988); Orchid Biosciences (IPO 2000); Acuity Pharmaceuticals (now OPKO Health, 2007); and NuMe Health (founded 2010, now MicroBiome Therapeutics). Dale is also the US-based General Partner of the London venture capital firm, Advent Life Sciences. Dale holds a BS degree from the University of CA and a PhD in physics from Brown University.
Dale Pfost, PhD, has 25 years of experience as a serial entrepreneur and executive in life sciences. He has been the founding CEO of 5 biotechnology companies – achieving 3 IPOs, 3 trade-sales and 3 companies with market capitalizations of over $2 billion. These include: Oxford GlycoSciences (IPO 1988); Orchid Biosciences (IPO 2000); Acuity Pharmaceuticals (now OPKO Health, 2007); and NuMe Health (founded 2010, now MicroBiome Therapeutics). Dale is also the US-based General Partner of the London venture capital firm, Advent Life Sciences. Dale holds a BS degree from the University of CA and a PhD in physics from Brown University.









