Dermatitis Herpetiformis : Familiar and Unknown
Nadia Arora, ND
Dermatitis herpetiformis (DH) is a clinical entity that has been revisited and characterized many times yet remains elusive. The disease was first described and named in 1884 by Dr Louis Duhring at the University of Pennsylvania, Philadelphia. It is a bullous-type dermatitis, extremely pruritic, and uncomfortable.

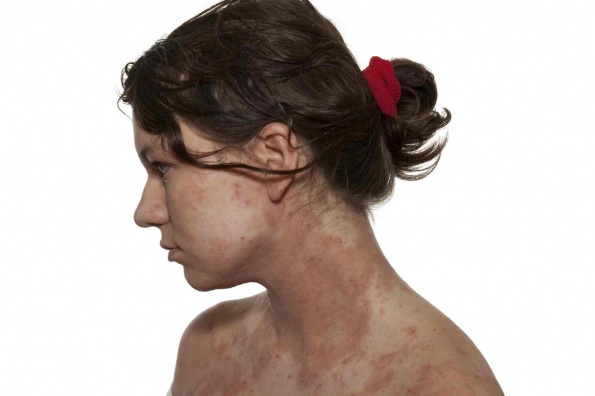
Classic DH typically manifests on extensor surfaces, such as elbows, knees, lower back, and shoulders, whereas atypical DH can present virtually anywhere, including the scalp, face, back of the neck, and along the hairline.1-3 As such, atypical DH is often misdiagnosed as eczema, psoriasis, or herpes simplex eruptions.
The course of the disease is similar to that of herpes: several hours before eruptions, there may be an intense burning and “prickling” sensation in the affected areas. Once the lesions appear, they look like small flesh-colored papules or vesicles, filled with fluid. Lesions of DH typically occur in groups and cause intense itching. The desire to scratch the lesions can be overwhelming, causing an affected individual to scratch the lesions until they bleed and form crusts.
There is controversy about the chronicity and progression of DH. Clearly, DH is a chronic disease, but in some patients it has a continuous and more severe character, while in others it seems to be more sporadic and relapsing-remitting in nature. A sporadic type frequently has a classic presentation: itching and burning are usually the first symptoms and may occur up to 12 hours before the lesions become visible.4 After the blisters develop, itching and burning may persist for up to 10 days, when they finally begin to dry and crust over. In patients with sporadic DH, misdiagnosis is rare, whereas in patients with continuous-type DH, it is more likely to be mistaken for eczema or psoriasis.
The common age at onset for DH is 30 to 40 years, although childhood onset or late onset (>60 years) is not uncommon. It is generally accepted that DH affects more men than women, particularly in adulthood, suggesting that hormonal factors may modify the course of the disease.5
Increased body mass index (BMI) was found to be positively related to DH in a 2009 study6 from Naples, Italy. Among 223 men and 924 women aged 20 to 60 years with untreated celiac disease (CD), the most common skin disease was DH. A 3.5 increase in BMI (calculated as weight in kilograms divided by height in meters squared) was associated with an excess prevalence of DH and was linear over the whole BMI range.
Is DH a Separate Entity or a Cutaneous CD?
Most patients with DH have clinical features of enteropathy that are similar to those of CD. Among patients with classic CD, DH is found in approximately 10% to 25%.7-9
On biopsy specimens, many patients with DH have patchy mucosal changes, although they are less severe than those associated with untreated CD. Some patients have no intestinal changes at all, but a certain degree of malabsorption is present in most individuals with DH. It is incompletely understood whether DH is a cutaneous manifestation of CD or is a separate entity, accompanied by a gluten-related enteropathy. Research tends to suggest that DH is a separate entity of an autoimmune cutaneous disease that appears as a manifestation of gluten intolerance; therefore, it belongs to a group of disorders that have gluten sensitivity in common, such as CD and gluten ataxia.3,10,11
On the other hand, there seems to be a consensus that gluten is the trigger of DH, similar to CD. Therefore, complete elimination of topical gluten and a lifelong gluten-free diet is usually recommended for affected individuals.
Course of DH
The course of the disease and its severity can vary over time, even in the same individual. That probably correlates with immune status and the amount of gluten in the diet. In my clinical experience, DH can be persistent or episodic, depending on the severity of a patient’s sensitivity to gluten.
Persistent severe DH is often the only initial symptom of atypical CD. Atypical CD manifests without apparent gastrointestinal symptoms, and skin symptoms may be the only clue to diagnosis, as illustrated by a retrospective analysis from Germany. The study12 looked at 32 patients who were treated for chronic DH from 1996 to 2008. All patients demonstrated skin lesions on the knees, elbows, gluteal region, and scalp. The ratio of male to female patients was 1.5:1, and the average age of the participants was 43 years. The interval between the first symptoms and diagnosis ranged from 6 weeks to 20 years. On testing, granular IgA deposits were found in the skin of affected individuals. Results of small intestinal biopsies were available from 29 patients and confirmed the presence of CD in all cases. Of note is the fact that no patient reported gastrointestinal symptoms. On serum testing, IgA antibodies against tissue transglutaminase and epidermal transglutaminase were found in 88% and 94% of patient serum samples, respectively. The authors concluded that patients with DH usually do not show apparent gastrointestinal symptoms, and in such cases skin lesions could be indicative of atypical CD and warrant further investigation.
Genetic Association
Many autoimmune conditions, such as thyroiditis, type 1 diabetes mellitus, rheumatoid arthritis, and sarcoidosis, have a strong genetic association with HLA class II genes. Similar to CD, DH is strongly associated with HLA-DQw2 and HLA-DR3 genotypes, according to a study13 from the United Medical School in London, England. In that study, HLA-DR, -DQ, and -DP genotyping was undertaken in 23 patients with DH and in 53 healthy control subjects. HLA-DQw2 was present in 100% (23 of 23) of patients with DH vs in 40% (21 of 53) of control subjects. Significant secondary associations occurred with HLA-DR3 (in 91% of patients with DH vs 28% of control subjects) and with HLA-DPw1 (in 39% of patients with DH vs 11% of control subjects). The authors concluded that DH and CD share an identical HLA class II association and proposed that these genes directly influence the immune responses that lead to mucosal damage in both diseases.
Of note are some genetic association studies that link DH to non-HLA genes, such as the haptoglobin gene. Haptoglobin is a blood plasma protein that binds free hemoglobin that is released from red blood cells. According to researchers from Hungary, polymorphism Hp2-1 in the haptoglobin gene is associated with significant risk for gluten-related enteropathy and its clinical presentations, such as DH.14 In the study, Hp2-1 was associated with a significant risk for CD (prevalence of 56.9% in patients vs 46.1% in controls) and was overrepresented among patients with mild symptoms (69.2%) or with silent disease (72.5%). Notably, Hp2-2 was less frequent in patients than in healthy controls, but patients having this phenotype were at an increased risk for severe malabsorption.
A weak association between DH and myosin IXB gene variants was found in a 2008 study from Finland.15 Myosin genes comprise a large family of genes whose protein products share the basic properties of actin binding, adenosine triphosphate (ATP) hydrolysis (ATPase enzyme activity), and force transduction. It is unclear how polymorphisms in the myosin gene confer susceptibility to DH, and further studies are needed to elucidate this mechanism.
Pathogenesis of DH
In DH, the deposition of IgA in the papillary dermis and at the dermoepidermal junction triggers an immunologic cascade, resulting in neutrophil recruitment and complement activation. The finding of granular deposits of IgA along the dermoepidermal junction is pathognomonic of DH, and multiple endogenous (genetic and hormonal) and exogenous (gluten and stress) factors are involved in this mechanism. Evidence is showing that epidermal transglutaminase 3 (eTG) is the dominant autoantigen of DH.12,16-18 Epidermal transglutaminase is a cytosolic enzyme involved in keratinocyte differentiation; therefore, both IgA and IgG against eTG lead to an inflammatory cascade that results in the formation of bullous lesions of the skin.
Epidermal transglutaminase is genetically and molecularly similar to tissue transglutaminase, an enzyme that is found in the gut. In most cases, patients with gluten-sensitive enteropathy have IgA antibodies to both epidermal and gut-type transglutaminase, regardless of whether they have DH.19 Levels of circulating antibodies have been found to correlate with each other, and both seem to correlate with the extent of gut disease.20 IgA-eTG complexes have been found in the dermis of patients with DH and, to a lesser extent, in unaffected skin of gluten-sensitive patients.21 Epidermal transglutaminase has not been observed in the dermis of control subjects without gluten sensitivity, supporting the leading theory of DH—that a genetic predisposition for gluten sensitivity, coupled with a diet high in gluten, leads to cutaneous autoimmune reaction and formation of the bullous lesions of DH.
Exacerbation of DH has been linked to certain exogenous factors, such as hormone-containing medications. There have been several clinical descriptions of DH induced by gonadotropin-releasing hormone analogues22 and by leuprolide acetate.23 Exacerbation of DH by progesterone-containing oral contraceptives has also been reported.24
Associated Conditions
Similar to CD, DH has been found to be associated with several autoimmune conditions, such as autoimmune thyroiditis, type 1 diabetes mellitus, and Sjögren syndrome. However, it is unclear whether such a comorbidity relates to polymorphisms in HLA genes, which many of the conditions share, or depends on the amount of gluten ingested. A 1997 Finnish study looked at the occurrence of associated diseases in a cohort of 305 patients with DH who were followed up for a mean of 10 years.25 The results were compared with those of 383 patients with CD. Twenty-nine patients with DH (9.5%) and 73 patients with CD (19.1%) had concomitant endocrine or connective tissue disorders. The incidences of associated conditions in patients with DH and in patients with CD were as follows: autoimmune thyroid disease in 4.3% and 6.0%, respectively; type 1 diabetes mellitus in 1.0% and 5.5%; lupus erythematosus in 1.3% and 0.3%; Sjögren syndrome in 1.0% and 2.9%; and sarcoidosis in 1.3% and 1.8%. Notably, the incidences of vitiligo and alopecia areata were 1.6% in patients with DH and 0% in patients with CD. Among the study cohort, most of these diseases manifested before DH had been diagnosed, suggesting that gluten withdrawal might be beneficial and prevent not only progression of the gluten-related disease but also development of other seemingly unrelated autoimmunities.
Treatment
Conventionally accepted treatments for DH involve a strict gluten-free diet and dapsone (5% gel) applications. Topical treatments are used for symptom relief only and do not address the underlying autoimmune processes associated with gluten intolerance; therefore, gluten elimination is mandatory to prevent further complications.
Response to treatment varies and depends on multiple factors, such as age, stage of the disease at diagnosis, extent of intestinal damage, and general state of health. Studies26,27 show that mucosal changes in DH, including oral mucosal lesions, respond well to a gluten-free diet, but skin response is slow and frequently incomplete. IgA and IgG antibodies to eTG tend to disappear after long-term (≤10 years) avoidance of dietary gluten.28,29
My clinical observation is that gluten-sensitive individuals frequently have outbreaks of DH as a reaction to increased gluten consumption, such as during holidays. In some patients on a gluten-free diet, the sudden appearance of DH is frequently the first and almost immediate symptom of relapse and can serve as an indicator that a strict gluten-free diet must be resumed. In many such cases, dermatitis resolves as gluten consumption decreases. However, the appearance of DH in some patients could indicate progression of gluten sensitivity to autoimmune CD.
Is Cure Possible?
Dermatitis herpetiformis can go into remission, according to researchers at the National Institutes of Health.30 That study provides insight into the pathogenesis and course of the disease and may guide long-term management of patients with DH. Among 86 patients studied, the disease remitted in 10 of them. A factor positively associated with DH remission, other than a prolonged gluten-free diet, was older age at onset (≥39 years) vs age at onset between 8 and 38 years. The authors suggested that clinicians should attempt to wean patients with well-controlled DH from a gluten-free diet and/or the use of sulfones or other therapies if it is determined that DH may have remitted.
Summary
Dermatitis herpetiformis is an autoimmune condition of the skin. It can be induced by gluten intake in genetically susceptible individuals. Although it can exist as a component of CD or as a separate entity, some degree of enteropathy is present with DH. Clinical signs can vary, ranging from grouped papules, vesicles with excoriations, or eczema-like lesions to psoriasis-like plaques, erythematosus or purpuric lesions, or acne-like pustules. A correct diagnosis depends on direct fluorescence studies of affected skin displaying granular IgA deposits in the dermis. Suspecting and then searching for DH is often clinically challenging, as the disease is a true chameleon with many clinical faces. Dapsone therapy alleviates the cutaneous symptoms and signs but does not prevent the systemic complications of enteropathy; therefore, strict adherence to a gluten-free diet is advisable. From the naturopathic viewpoint, a gluten-free diet is the best and lowest-force intervention available today for the treatment of DH and for the prevention of further complications.
Dr. Nadia Arora, ND is a licensed naturopathic physician in Washington DC. She graduated from Bastyr University in Seattle with the degree of Doctor of Naturopathic Medicine. Dr. Arora is a primary care physician with the specialized training in natural medicine. Her focus areas are digestive health and dermatology.
Dr. Arora also serves on the board of directors for Kromosoft, a genetics research company based in Virginia. She directs the company’s research department and is a medical writer for their weekly newsletter, KromoNews. She is the author of more than 30 articles on the topic of human hereditary diseases and genetics of cancer.
Since 2006, Dr. Arora serves on the medical advisory board for the Spinal Cord Tumor Association. She is a regular speaker at the SCTA biennial conferences, where she presents her findings about the newest evidence-based naturopathic, immunologic and biologic treatments for cancer.
References
1. McGovern T, Bennion SD. Palmar purpura: an atypical presentation of childhood dermatitis herpetiformis. Pediatr Dermatol. 1994;11(4):319-322.
2. Powell GR, Bruckner AL, Weston WL. Dermatitis herpetiformis presenting as chronic urticaria. Pediatr Dermatol. 2004;21(5):564-567.
3. Woollons A, Darley CR, Bhogal BS, Black MM, Atherton DJ. Childhood dermatitis herpetiformis: an unusual presentation. Clin Exp Dermatol. 1999;24(4):283-285.
4. Hall RP. The pathogenesis of dermatitis herpetiformis: recent advances. J Am Acad Dermatol. 1987;16(6):1129-1144.
5. Bardella MT, Fredella C, Saladino V, et al. Gluten intolerance: gender- and age-related differences in symptoms. Scand J Gastroenterol. 2005;40(1):15-19.
6. Zingone F, Bucci C, Tortora R, et al. Body mass index and prevalence of skin diseases in adults with untreated coeliac disease. Digestion. 2009;80(1):18-24.
7. Herrero-Gonzáles JE. Clinical guidelines for the diagnosis and treatment of dermatitis herpetiformis [in Spanish]. Actas Dermosifiliogr. 2010;101(10):820-826.
8. Kárpáti S. Dermatitis herpetiformis: close to unravelling a disease. J Dermatol Sci. 2004;34(2):83-90.
9. Biagi F, Corazza GR. Mortality in celiac disease. Nat Rev Gastroenterol Hepatol. 2010;7(3):158-162.
10. Cuartero BG, Santamaría MJ, Acuña Quirós MD, et al. Dermatitis herpetiformis vs. celiac disease [in Spanish]. An Esp Pediatr. 1992;37(4):307-310.
11. Pfeiffer C. Dermatitis herpetiformis: a clinical chameleon [in German]. Hautarzt. 2006;57(11):1021-1029.
12. Rose C, Bröcker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring [in English and German]. J Dtsch Dermatol Ges. 2010;8(4):265-271.
13. Hall MA, Lanchbury JS, Bolsover WJ, Welsh KI, Ciclitira PJ. HLA association with dermatitis herpetiformis is accounted for by a cis or transassociated DQ heterodimer. Gut. 1991;32(5):487-490.
14. Papp M, Foldi I, Nemes E, et al. Haptoglobin polymorphism: a novel genetic risk factor for celiac disease development and its clinical manifestations. Clin Chem. 2008;54(4):697-704.
15. Koskinen LL, Korponay-Szabo IR, Viiri K, et al. Myosin IXB gene region and gluten intolerance: linkage to coeliac disease and a putative dermatitis herpetiformis association. J Med Genet. 2008;45(4):222-227.
16. Asano Y, Makino T, Ishida W, Furuichi M, Shimizu T. Detection of antibodies to epidermal transglutaminase but not tissue transglutaminase in Japanese patients with dermatitis herpetiformis [published online ahead of print November 29, 2010]. Br J Dermatol. doi:10.1111/j.1365-2133.2010.10153.x. Medline:21114479.
17. Rose C, Bröcker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring [in English and German]. J Dtsch Dermatol Ges. 2010;8(4):265-271.
18. Rose C, Armbruster FP, Ruppert J, Igl BW, Zillikens D, Shimanovich I. Autoantibodies against epidermal transglutaminase are a sensitive diagnostic marker in patients with dermatitis herpetiformis on a normal or gluten-free diet. J Am Acad Dermatol. 2009;61(1):39-43.
19. Kumar V, Jarzabek-Chorzelska M, Sulej J, Rajadhyaksha M, Jablonska S. Tissue transglutaminase and endomysial antibodies: diagnostic markers of gluten-sensitive enteropathy in dermatitis herpetiformis. Clin Immunol. 2001;98(3):378-382.
20. Jaskowski TD, Hamblin T, Wilson AR, et al. IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis and pediatric celiac disease. J Invest Dermatol. 2009;129(11):2728-2730.
21. Hall RP, Lawley TJ, Katz SI. Dermatitis herpetiformis. Springer Semin Immunopathol. 1981;4(1):33-43.
22. Yu SS, Connolly MK, Berger TG, McCalmont TH. Dermatitis herpetiformis associated with administration of a gonadotropin-releasing hormone analog. J Am Acad Dermatol. 2006;54(2)(suppl):S58-S59.
23. Grimwood RE, Guevara A. Leuprolide acetate–induced dermatitis herpetiformis. Cutis. 2005;75(1):49-52.
24. Hassan S, Dalle S, Descloux E, Balme B, Thomas L. Dermatitis herpetiformis associated with progesterone contraception [in French]. Ann Dermatol Venereol. 2007;134(4, pt 1):385-386.
25. Reunala T, Collin P. Diseases associated with dermatitis herpetiformis. Br J Dermatol. 1997;136(3):315-318.
26. Reunala T, Kosnai I, Karpati S, Kuitunen P, Török E, Savilahti E. Dermatitis herpetiformis: jejunal findings and skin response to gluten free diet. Arch Dis Child. 1984;59(6):517-522.
27. Cooper BT, Mallas E, Trotter MD, Cooke WT. Response of the skin in dermatitis herpetiformis to a gluten-free diet, with reference to jejunal morphology. Gut. 1978;19(8):754-758.
28. Fry L. Dermatitis herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12(6):523-531.
29. Reunala T. Dermatitis herpetiformis: coeliac disease of the skin. Ann Med. 1998;30(5):416-418.
30. Paek SY, Steinberg SM, Katz SI. Remission in dermatitis herpetiformis: a cohort study. Arch Dermatol. 2011;147(3):301-305.