Stress-Induced Hair Loss: Benefits from a Standardized Nutraceutical
Vis Medicatrix Naturae
MELISSA ANZELONE, ND
ALEKSANDER RICHARDS
GIORGIO DELL’ACQUA, PHD
It is estimated that 35-million men and 21-million women suffer from some degree of hair loss in the United States.1 By 35 years of age, 40% of men experience hair loss, and this increases to 70% by age 80.1 Similarly, approximately 80% of women have hair loss by age 60.1 The negative psychosocial impact of hair loss,2 including its effect on quality of life, is well known3 and generally observed to be more severe among women than men.4,5
Hair production occurs in an ongoing cycle that includes: growth (anagen phase), regression (catagen phase), rest (telogen phase), and, finally, release from the follicle and shedding from body (exogen phase). During each anagen phase, follicles produce an entire hair shaft, from tip to root. During catagen and telogen phases, follicles reset and prepare their stem cells so that they can receive the signal to start the next growth phase and make a new hair shaft.6 However, this normal cycle is easily disrupted by chronic stress, as we shall see in the patient case discussed below.
Stress-Related Hair Loss
Effect of Stress on Hair Growth
Stress is recognized as an underlying cause of many illnesses. It is estimated that 75-90% of all human disease is related to stress.7 A chronic proinflammatory response is an essential component of disease, and the stress response is an important adaptive psychophysiological survival mechanism in the face of an acute stress. However, the so-called fight-or-flight response is often misdirected in the modern age. Rather than primarily being used to fight or escape predators or enemies, the stress response is rerouted in ways that trigger or worsen mechanisms that can lead to illness.
The stress hormone, cortisol, is known to affect the function and cyclic regulation of the hair follicle.8 During periods of stress, cortisol dysregulation, an increased proinflammatory response, disrupted cell signaling, and oxidative stress can all disrupt the normal transitions of the hair growth cycle. In-vitro studies have demonstrated that proinflammatory cytokines, including tumor necrosis factor-alpha (TNFα), interleukin (IL)-1α, and IL-1β, cause the formation of vacuoles within hair matrix cells as well as abnormal keratinization of the inner root sheath and follicle bulb and inner root sheath; they have also been shown to disrupt follicular melanocytes and promote the formation of melanin granules within the dermal papilla.9 Proinflammatory cytokines can disturb the hair cycle, which may cause premature arrest of hair cycling.10 Specifically, these molecules can prevent the hair growth cycle from moving from the telogen phase to the anagen growth phase.11 Mouse studies have also shown chronic stress to inhibit hair growth, increase mast cell granulation, and promote inflammation around hair follicles.12 Further studies, both in vitro and in vivo, have demonstrated that certain stress-mediating substances, such as substance P, ACTH, and cortisol, can inhibit hair growth.8,13-15
As an example of a stress-related hair-loss condition, telogen effluvium (TE) is characterized by a non-scarring, non-inflammatory alopecia of relatively sudden onset, caused by physiologic or emotional stress.11 A recently published multi-center study, evaluating almost 3000 patients across several countries, identified TE as the third cause of alopecia after androgenic alopecia and alopecia areata.16
Alopecia areata (AA) is an inflammatory autoimmune disease that involves non-scarring hair loss. Often it presents as clearly circumscribed patches of hair loss on the scalp, though it can progress to the point of involving the entire scalp. Histologically, AA includes an acute stage, involving an attack by auto-aggressive T-cells on the anagen hair follicles. The etiology of AA may stem from emotional and environmental stressors as well as a decrease in antioxidant/oxidant balance17; genetic predisposition can also play a role in AA18
Diagnosis of Stress-Related Hair Loss
Historically, conventional medicine has tried to address single causes of hair loss, such as hormones or genetics. In fact, hair loss has many root causes, including nutrition, hormones, stress, metabolism, and environmental factors. All of these attributes should be evaluated when diagnosing the causes of hair loss. Several patient characteristics, as outlined below, can be specifically attributed to stress.
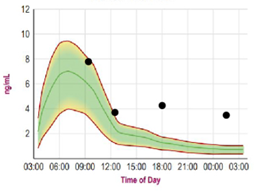
Saliva cortisol testing is a non-invasive collection method whereby saliva is collected in plastic tubes and analyzed using liquid chromatography/tandem mass spectrometry19; it may well become one of the gold standards for assessing stress hormones.20 This method is frequently used as a diagnostic tool to identify chronic stress and adrenal fatigue. Given its success in tracking stress levels, it may also prove useful in understanding stress-related hair thinning. Salivary measurement of cortisol has several advantages over cortisol measurement in the blood. For example, most steroid hormones in the bloodstream are bound to carrier proteins and therefore unavailable to target tissues. Serum measurement reflects both bound and unbound cortisol, whereas salivary testing reflects only the fraction of unbound, bioavailable hormone.21 Saliva is also preferred to serum in situations where venipuncture stress can affect results. Finally, cortisol levels vary over the course of the day.22,23 Because of the ease of collection, saliva is the method of choice for biobehavioral research on hormones and for diurnal cortisol testing, since the requirement of multiple collections makes serum testing inconvenient. In Figures 1a and 1b, the green-shaded area represents the normal range, the red line defines the upper and lower limits, and the green line represents mean values.
Treatment of Stress-Related Hair Loss
Conventional methods of treating telogen effluvium (TE) and AA include both topical and injectable corticosteroids, which can provide rapid results but possibly be associated with fat and skin atrophy.24 Other topical pharmaceutical treatments include ketoconazole shampoo and vasodilators such as minoxidil.25 These also produce satisfactory results in some patients but can be associated with adverse events.
Alternative interventions, such as the use of nutraceuticals, can target the multifactorial causes of poor hair health. Nutraceutical approaches can also frequently help patients to better manage anxiety and stress, promote better sleep, and improve skin and nail health.26 By rebalancing the stress and proinflammatory pathways that target the hair follicle, a combination of botanicals with both adaptogenic and antioxidant properties, such as those listed in Table 1, may offer protection against this major causal factor of hair thinning.8,16,27 The effect of curcumin on TNFα and IL-1 may be particularly noteworthy, since these cytokines have been shown to interfere with hair growth.10
Table 1. Antioxidant & Adaptogenic Botanical Formula
| Botanical | Phytoactive Component | Main Physiological Actions |
| Curcumin (Curcuma longa) | Extract standardized to 95% curcuminoids | Reduces proinflammatory compounds: ↓ COX-2, lipoxygenases, NOS, NF-ĸB, TNFα, IL-116,28,29 |
| Ashwagandha (Withania somnifera) | Extract standardized to 10% withanolide glycosides | Adaptogenic botanical Affects HPA axis: ↓ anxiety, cortisol, mean serum CRP concentration30 |
| Japanese Knotweed (Polygonum cuspidatum) | Extract standardized to 50% resveratrol | Antioxidant and adaptogenic31: ↓ TNFα induced MCP-1, NF-ĸB activity32 |
Case Report
A 35-year-old woman presented in 2017 with a complaint of a bare spot at the vertex of her head where she parts her hair. She described having an “aggressive lifestyle,” liking to “work and play hard.” In addition, she had just completed an egg cryopreservation cycle the previous month, which she said was very stressful, both emotionally and physically. Prior to consulting me, she saw both a naturopathic doctor and an integrative medical doctor for a complete medical workup. She reported poor sleep hygiene habits, including working on her laptop in bed until 4 AM or “pulling all-nighters,” where she would stay out late to socialize and then immediately work a full day and night afterwards. She said she would sleep only 1-2 hours per night. She had been prescribed adrenal support supplements in the past, and at the time of her first visit in 2017 she was taking vitamin B complex, fish oil, a multivitamin, magnesium glycinate, and a variety of probiotics. She followed a dairy- and gluten-free diet.
A direct-to-consumer salivary cortisol test was administered twice: prior to treatment in order to obtain a baseline, and then again 10 months later after a noticeable change in hair quality and quantity was observed. Four tubes of saliva were collected: 1 upon waking, 1 before lunch, 1 before dinner, and 1 at bedtime. Exact times of collection were recorded on each tube, and all tubes were labeled.
Her baseline salivary test revealed elevated cortisol levels at all collection time-points (Figure 1a). The 9 AM reading was high-normal, at 8 ng/mL; her 12 PM reading was also high-normal, at 4 ng/mL. Gross elevations were apparent at 6 PM (18:00 hr on the graph) and 2 AM, at 4 ng/mL and 3.8 ng/mL, respectively. These levels were consistent with chronic stress.
Figure 1a. Salivary Cortisol: Pre-Intervention
The subject was instructed to take 4 capsules per day of a proprietary nutraceutical formula, the primary ingredients of which were Curcuma longa, Withania somnifera, and Polygonum cuspidatum (Table 1).26
Digital images of the patient were also obtained every 3 months to monitor progress. Figure 2a shows her scalp prior to treatment. She took the nutraceutical as instructed, and she regularly reported any changes in her hair and symptoms during the course of treatment.
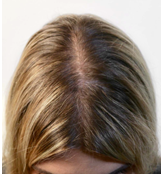
After 10 months of the intervention, her salivary cortisol levels were substantially reduced. A post-treatment image was obtained (Figure 2b), which demonstrated improvement in the area of thinning hair on her scalp. She also reported an overall improvement in hair quality and shine.
Figure 1b: Salivary Cortisol: Post-Intervention
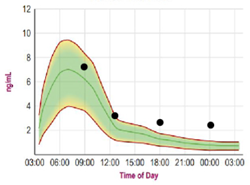
Figure 2a. “Before” Photo
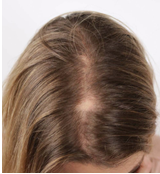
Figure 2b. “After” Photo
Conclusion
This case report demonstrates the effect of chronic stress on hair loss, its correlation with elevated salivary cortisol levels, and clinical improvement following 10 months of a nutraceutical formulation. Moreover, the clinical improvement correlated with a decrease in cortisol levels, suggesting salivary cortisol may be a useful tool in both diagnosis and monitoring of stress-related hair loss. An adaptogenic and antioxidant nutraceutical proved to be a positive intervention in terms of improving salivary cortisol levels as well as hair growth and quality of hair. Although these observations represent a single case report, they are promising, and further research is warranted to explore the relationship between hair growth and cortisol levels.
References:
- The Hair Society. Hair Loss Statistics. January 19, 2015. Available at: https://thehairsociety.org/hair-loss-statistics-the-facts. Accessed March 20, 2020.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21(11):1829-1836.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15(2):137-139.
- Davis DS, Callender VD. Review of quality of life studies in women with alopecia. Int J Womens Dermatol. 2018;4(1):18-22.
- Ahluwalia J, Fabi SG. The psychological and aesthetic impact of age-related hair changes in females. J Cosmet Dermatol. 2019;18(4):1161-1169.
- Panossian A, Gabrielian E, Wagner H. On the mechanism of action of plant adaptogens with particular reference to cucurbitacin R diglucoside. Phytomedicine. 1999;6(3):147-155.
- Liu YZ, Wang YX, Jiang CL. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci. 2017;11:316.
- Thom E. Stress and the hair growth cycle: cortisol-induced hair growth disruption. J Drugs Dermatol. 2016;15:1001-1004.
- Kasumagic-Halilovic E, Prohic A, Cavaljuga S. Tumor necrosis factor-alpha in patients with alopecia areata. Indian J Dermatol. 2011;56(5):494-496.
- Hoffmann R. The potential role of cytokines and T cells in alopecia areata. J Investig Dermatol Symp Proc. 1999;4(3):235-238.
- Rebora A. Telogen effluvium: a comprehensive review. Clin Cosmet Investig Dermatol. 2019;12:583-590.
- Krause K, Foitzik K. Biology of the hair follicle: the basics. Semin Cutan Med Surg. 2006;25(1):2-10.
- Choi SJ, Cho AR, Jo SJ, et al. Effects of glucocorticoid on human dermal papilla cells in vitro. J Steroid Biochem Mol Biol. 2013;135:24-29.
- Kwack MH, Lee JH, Seo CH, et al. Dickkopf-1 is involved in dexamethasone-mediated hair follicle regression. Exp Dermatol. 2017;26(10):952-954.
- Peters EM, Liotiri S, Bodó E, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171(6):1872-1886.
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the Types of Alopecia at Twenty-Two Specialist Hair Clinics: A Multicenter Study. Skin Appendage Disord. 2019;5(5):309-315.
- Prie BE, Voiculescu VM, Ionescu-Bozdog OB, et al. Oxidative stress and alopecia areata. J Med Life. 2015;8 Spec Issue:43-46.
- Jabbari A, Petukhova L, Cabral RM, et al. Genetic basis of alopecia areata: a roadmap for translational research. Dermatol Clin. 2013;31(1):109-117.
- Perogamvros I, Owen LJ, Keevil BG, et al. Measurement of salivary cortisol with liquid chromatography-tandem mass spectrometry in patients undergoing dynamic endocrine testing. Clin Endocrinol (Oxf). 2010;72(1):17-21.
- Soo-Quee Koh D , Choon-Huat Koh G. The use of salivary biomarkers in occupational and environmental medicine. Occup Environ Med. 2007;64(3):202-210.
- Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic-pituitary-adrenal axis activity. Clin Endocrinol (Oxf). 2005;63(3):336-341.
- Thau L, Sharma S. Cortisol Physiology. Treasure Island, FL: StatPearls Publishing; 2020.
- Adam EK, Quinn ME, Tavernier R, et al. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25-41.
- Park SK, Choi YS, Kim HJ. Hypopigmentation and subcutaneous fat, muscle atrophy after local corticosteroid injection. Korean J Anesthesiol. 2013;65(6 Suppl):S59-S61.
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341(13):964-973.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17(5):558-565.
- Farris PK, Rogers N, McMichael A, Kogan S. A novel multi-targeting approach to treating hair loss, using standardized nutraceuticals. J Drugs Dermatol. 2017;16(11):s141-s148.
- Tavakoli J, Miar S, Majid Zadehzare M, Akbari H. Evaluation of effectiveness of herbal medication in cancer care: a review study. Iran J Cancer Prev. 2012;5(3):144-156.
- Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. 2011;12(3):332-347.
- Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):208-213.
- Peron G, Uddin J, Stocchero M, et al. Studying the effects of natural extracts with metabolomics: A longitudinal study on the supplementation of healthy rats with Polygonum cuspidatum Sieb. et Zucc. J Pharm Biomed Anal. 2017;140:62-70.
- Zhu J, Yong W, Wu X, et al. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun. 2008;369(2):471-447.

Melissa Anzelone, ND, is the Director of Education and Corporate Wellness at Nutrafol. Melissa obtained her doctorate degree in naturopathic medicine from the University of Bridgeport, CT. She was fortunate to spend her internship hours training with Drs Ashley ewin, Peter D’Adamo, and David Brady. Throughout the 10 years she has been in the naturopathic field, Melissa has exemplified her passion for natural health by helping clients balance hormones, intervening in metabolic disease, and promoting weight loss using the gentlest modalities first. In her free time, Melissa enjoys spending time with her husband, trying new restaurants, and sweating at her local Pilates studio.
***

Aleksander Richards graduated from Fordham University in 2016, with a bachelor’s degree in biology and pre-med. He has since been formulating and designing products that target specific pathways relating to hair loss, while helping to lead the R&D team. He is also currently working toward an MBA, with a concentration in entrpreneurship and innovation.
***

Giorgio Dell’Acqua, PhD, is the scientific director of Nutrafol, where he leads the innovation team to investigate new leads in hair health and hair growth. A graduate from the University of Rome, Italy, Dr Dell’Acqua worked for 15 years as an academic in applied medical research. He has spent the last 20 years as an executive and cosmetic scientist in the personal care industry. He has authored more than 70 publications in medicine and cosmetic science and is an award-winning speaker on natural ingredients. He also chairs the Scientific Committee for the New York Society of Cosmetic Chemists.