Therapeutic Psychobiotics: Modulating Mood and More via the Gut-Brain Axis
JESSICA BRANDES, ND
If microbes are controlling the brain, then microbes are controlling everything. (John F. Cryan)
According to a recent 3-part study conducted on behalf of the American Psychological Association,1-3 US adults surveyed reported elevated levels of stress that was exacerbated by a variety of recent events beyond the COVID pandemic. Seventy percent of those surveyed considered the economy a significant stressor (as compared to 69% during the 2008 recession); 83% considered the future of the nation a significant source of stress; and 60% were stressed by incidents of police violence involving minorities. Undoubtedly, these are unusually stressful times. As such, a review of the relationship between the GI microbiome and stress seems timely.
The Gut-Brain Axis
The so-termed gut-brain axis is a complex, bidirectional communications network inclusive of the central, autonomic, and enteric nervous systems, as well as the hypothalamic-pituitary-adrenal (HPA) axis. The sympathetic and parasympathetic nervous systems play roles in both afferent signaling – from the intestinal lumen to the central nervous system (CNS) via enteric, spinal, and vagal routes – and efferent signaling from the CNS to the intestines. In large part, the stress response plays out in the biofeedback loop of the HPA. It is activated by external stressors as well as endogenous proinflammatory cytokines. No matter the stimulus, it results in hypothalamic secretion of corticotropin-releasing factor (CRF), which leads directly to adrenal gland secretion of the stress hormone, cortisol. It is through these neuroendocrine pathways, along with enteric immune system signaling, that the cerebral and enteric brains communicate. Additionally, commensal bacteria within the gut microbiota influence the entire process.
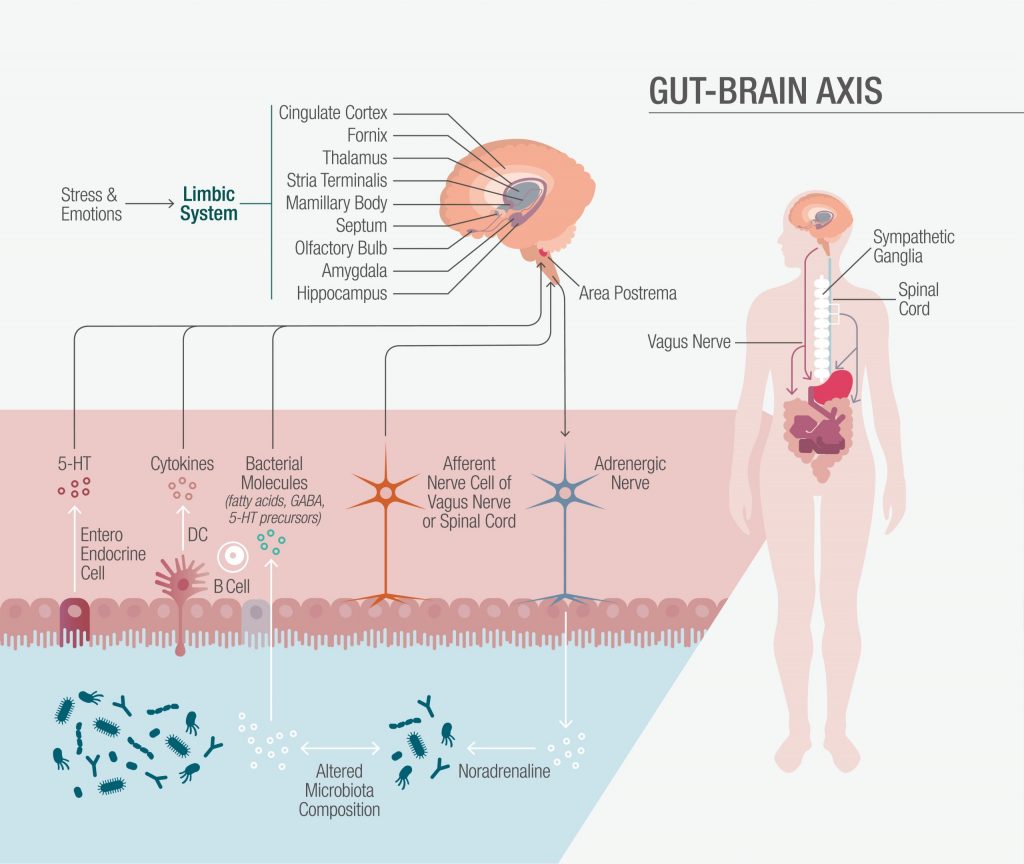
Although individual variations in stress response have been associated with both genetic and social/environmental factors, animal studies have shown that early exposure to stressful events, in particular, can have a lasting impact on the developing brain.4,5 Although beyond the scope of this article, research in recent years has also made it apparent that intestinal bacteria are active participants in early brain development as well as in neuroplasticity and neurogenesis into adulthood.6,7 Our focus here will be to review the mechanisms by which the gut influences the CNS and to examine the potential for probiotic interventions to positively impact mood.
Mechanisms & Mood
Although not entirely understood, microbiota bottom-up modulation of the CNS involves several mechanisms. The majority of the evidence elucidating the gut microbiota’s influence on mood and stress can be found in the preclinical work of leading gut-brain researchers.8,9
The Nervous System
The enteric nervous system typically regulates routine gastrointestinal functions including peristalsis and mucus secretion, but the CNS is capable of modulating local control through the autonomic nervous system and afferent vagal pathways. It has been hypothesized that an exaggerated stress response associated with functional gastrointestinal disorders may be related to altered stress processing in the CNS or peripherally in the enteric nervous system.10 Investigators have shown that mysenteric neurons of germ-free mice display reduced excitability, which is corrected when the animals are colonized with normal gut bacteria.11
Metabolites & Molecules
Microbial-derived metabolites, including short-chain fatty acids, interact with the enteric system through enteroendocrine and enterochromaffin cells; however, an animal model suggests that these substances can also cross into systemic circulation.12 Through channels that may involve directly crossing the blood-brain barrier and/or inducing afferent vagal and CNS signaling, the gut stays in constant communication with the brain. Moreover, commensal microbes are directly involved in the production of neuroactive substances, including gamma-aminobutyric acid (GABA), serotonin (5-HT), norepinephrine, and dopamine.
Notably, germ-free mice demonstrate significant alterations in normal hypothalamic neurotransmitters, including serotonin,6 that are integral to CNS development and plasticity.13
Additional work with germ-free mice has demonstrated the ability of Bifidobacterium to influence central GABA receptor expression and related regulation of emotional behavior,14 modulate stress-like behavior,15 and have anxiolytic effects.16
Pathogen Inhibition
Commensal organisms in the GI lumen can effectively compete with pathogens for resources in the gut, thereby preventing colonization. Perturbations in the normal gut flora, as a result of diet, medications, or other factors, allow pathogens to take up residence in the intestinal epithelium. Resulting toxins and local inflammation can then damage actin filaments, leading to intestinal hyperpermeability.17 More specifically, the gram-negative endotoxin, lipopolysaccharide (LPS), induces proinflammatory cytokines within the intestinal lumen and activates microglial toll-like receptors, leading to the release of proinflammatory cytokines in the CNS. Not only has LPS has been implicated in depressive and anxious behavior in mice with intact vagal nerves,18 anti-LPS IgA and IgM antibodies have also been found in the serum of both depressed patients and those diagnosed with chronic fatigue syndrome.19
Intestinal Barrier Integrity
Excessive intestinal permeability, or so-called “leaky gut,” has a demonstrable impact on the gut-brain axis. Translocation of intestinal bacteria and LPS through impaired tight junctions results in the release of proinflammatory cytokines and vagal activation, which in turn leads to HPA-activated cortisol release. Animal models have shown that exposure to stress significantly changes the microbiome makeup, such as a decrease in Bacteroides and an increase in Clostridium.20 Further research showed that feeding mice probiotic species capable of influencing tight junctions resulted in improved intestinal barrier integrity and decreased HPA-axis activation.21
The Potential of Therapeutic Psychobiotics
Psychobiotics have been defined as beneficial bacteria (probiotics), or support for such bacteria (prebiotics), that influence bacteria-brain relationships. These substances exert anxiolytic and antidepressant effects characterized by changes in cognitive, emotional, systemic, and neurological indices.22
Beyond a large body of animal gut-brain research, there are several recent human trials evaluating the impact of probiotic species in the stress response (see Table 1).
It is important to note that differences in strain selection, study design, and primary outcomes make it difficult to draw clear conclusions about general probiotic efficacy. That said, the potential impact of psychobiotics is quite promising. There are, however, a number of key areas of consideration when choosing a probiotic to support the gut-brain axis. The 2 primary areas for consideration when selecting a targeted probiotic include formulation evidence and manufacturing quality, each of which is briefly discussed below.
Design | Probiotic Studied | Outcomes | Year | Citation |
| Double-blind, placebo-controlled study investigated a probiotic’s anxiolytic properties in rats, as well as its effects on depression, anxiety, and stress in healthy humans. Assessments: Hopkins Symptom Checklist (HSCL-90); Hospital Anxiety and Depression Scale (HADS); Perceived Stress Scale; Coping Checklist (CCL); 24-h urinary free cortisol | Lactobacillus helveticus R0052; Bifidobacterium longum R0175 | The probiotic alleviated psychological distress in human subjects, particularly as measured by the HSCL-90 scale, the HADS, and by the CCL. | 2010 | Messaoudi M, Violle N, Javelot H, et al. Br J Nutr. 2011;105(5):755-764. |
| Clinical study randomized 36 healthy women into 3 groups. Participants were given a fermented milk product with probiotic (FMPP) (n=12); a non-fermented milk product (n=11, controls); or no intervention (n=13), twice daily for 4 wk. Assessments: fMRI before and after the intervention, to assess brain response to an emotional faces attention task and resting brain activity | Bifidobacterium animalis subsp lactis I-2494; Streptococcus thermophilus I-1630; Lactobacillus bulgaricus I-1632/I-1519; Lactococcus lactis subsp lactis I-1631 | The FMPP group showed reduced activity in sensory brain regions, as well as in frontal, prefrontal, and temporal cortices, parahippocampal gyrus, and the periaqueductal gray. | 2013 | Tillisch K, Labus J, Kilpatrick L, et al. Gastroenterology. 2013;144(7):1394-1401. |
| Triple-blind, placebo-controlled, randomized, pre- and post-intervention study assessed the effects of a multi-species probiotic on cognitive reactivity to sad mood in 20 healthy participants over 4 wk. Assessments: The revised Leiden index of depression sensitivity scale | Bifidobacterium bifidum W23; B lactis W52; Lactobacillus acidophilus W37; L brevis W63; L casei W56; L salivarius W24; Lactococcus lactis W19/W58 | The probiotic group showed reduced overall cognitive reactivity to sad mood, mostly in the form of reduced rumination and aggressive thoughts. | 2015 | Steenbergen L, Sellaro R, van Hemert S, et al. Brain Behav Immun. 2015;48:258-264. |
| Double-blind, placebo-controlled pilot study examined the effects of a probiotic on physiological and psychological stress responses in healthy medical students scheduled to take a nationwide exam. Participants were given either a probiotic (n=24) or placebo (n=23). Assessments: Psychophysical state; fecal serotonin; salivary cortisol; plasma tryptophan. Measurements were taken at intervals between 8 wk prior to testing and 2 wk afterward. | Lactobacillus casei strain Shirota (LcS) | One day before the exam, cortisol and tryptophan were elevated only in the placebo group. Two days after the exam, fecal serotonin was more elevated in the probiotic group. During the pre-exam period, physical stress symptoms were lower in the probiotic group. Results suggested the probiotic could help prevent physical symptoms due to stress. | 2016 | Kato-Kataoka A, Nishida K, Takada M, et al. Benef Microbes. 2016;7(2):153-156. |
| Randomized, placebo-controlled, crossover study examined the effects of a probiotic on stress response in 29 healthy men over 8 wk. Assessments: Self-reporting of stress measures; cognitive assessments; and resting EEG. Also plasma IL-10, IL-1β, IL-6, IL-8, and TNFα; whole-blood TLR-4 agonist-induced cytokine release; salivary cortisol and subjective stress measures were evaluated before, during and after a socially evaluated cold pressor test (SECPT). | Lactobacillus rhamnosus JB1 | The probiotic had no overall effect on mood, anxiety, sleep quality, or stress or on the HPA response to the SECPT. Compared to placebo, the probiotic produced no improvements in visuospatial memory performance, attention switching, rapid visual information processing, emotion recognition and associated EEG measures. No significant anti-inflammatory differences were observed in basal and stimulated cytokines. | 2016 | Kelly JR, Allen AP, Temko A, et al. Brain Behav Immun. 2017;61:50-59 |
| Double-blind, randomized, placebo-controlled study examined the effect of a probiotic on digestive health (primary outcome) and well-being (secondary outcome) in 290 older adults (>65 years). Participants were given either the probiotic or placebo. Assessments: Self-reported changes in GI symptoms, well-being, stress, and anxiety | Lactobacillus reuteri DSM 17938 | There were no significant persistent effects of the probiotic on the primary or secondary outcome measures. In the probiotic group, stress levels modestly decreased at wk 8 and 12. | 2016 | Östlund-Lagerström L, Kihlgren A, Repsilber D, et al. Nutr J. 2016;15(1):80. |
| A within-participants study examined the effects of a probiotic on stress response in 22 healthy subjects. Participants were screened at baseline, then given placebo for 4 wk, followed by the probiotic for 4 wk. Assessments: Daily online questionnaires; cognitive assessments, resting EEG, and cortisol at baseline, post-placebo, and post-probiotic in response to a SECPT | Bifidobacterium longum 1714 | Daily reported stress and increases in cortisol and subjective anxiety following the SECPT were all attenuated by probiotic administration. Visuospatial memory performance also improved after the probiotic. | 2016 | Allen AP, Hutch W, Borre YE, et al. Transl Psychiatry. 2016;6(11):e939 |
| Double-blind, randomized, placebo-controlled study examined the effect of a probiotic on mood, memory, and cognition in 79 subjects prone to depression but not currently taking psychotropic medications. Participants took either the probiotic or placebo for 8 wk. Assessments: Self-reported mood measurements; Montgomery-Asberg Depression Rating Scale; inflammatory and other biomarkers; vitamin D | Lactobacillus helveticus R0052; Bifidobacterium longum R0175 | The probiotic had no observable effect on mood or biomarkers compared to placebo. The lack of effect was thought to possibly be due to severity, chronicity, or resistance to treatment among participants. | 2017 | Romijn AR, Rucklidge JJ, Kuijer RG, et al. Aust N Z J Psychiatry. 2017;51(8):810-821. |
| Double-blind, placebo-controlled, randomized study investigated the effects of a multi-strain probiotic on cognitive function, behavior, and intestinal microbial composition in 49 healthy subjects. Participants were randomized equally to probiotic, placebo, or no intervention for 4 wk. Assessments: fMRI; Leiden Index of Depression; PANAS scoring; stool analysis for intestinal microbiota | Lactobacillus casei W56; L acidophilus W22; L paracasei W20; L salivarius W24; L plantarum W62; Lactococcus lactis W19; Bifidobacterium lactis W51; B lactis W52; B bifidum W23 | Probiotic administration was associated with self-reported improvements in mood and cognitive reactivity, memory (as revealed by brain activation patterns), and subtle changes in gut microbial composition. | 2018 | Bagga D, Reichert JL, Koschutnig K, et al. Gut Microbes. 2018;9(6):486-496. |
| Randomized, double-blind, placebo-controlled study examined the effect of a probiotic on mood, memory, and cognition in 103 stressed adults. Participants were given either probiotic (n=52) or placebo (n=51) for 12 wk. Assessments: DASS-42 questionnaire; plasma cortisol; proinflammatory cytokines | Lactobacillus plantarum P8 | Compared with placebo, probiotic administration was associated with reduced measures of stress and anxiety, as well as reductions in proinflammatory cytokines that were accompanied by improvements in memory and cognition. Plasma cortisol was only marginally different between probiotic and placebo groups. | 2019 | Lew LC, Hor YY, Yusoff NAA, et al. Clin Nutr. 2019;38(5):2053-2064. |
| Randomized, double-blind, placebo-controlled study examined the effect of a probiotic on stress, anxiety, and mood in 111 stressed adults. Subjects were given either probiotic (n=56) or placebo (n=55) for 12 wk. Assessments: DASS-42 questionnaire; plasma cortisol; proinflammatory cytokines; plasma neurotransmitter metabolites | Lactobacillus plantarum DR7 | Probiotic administration was associated with reduced symptoms of stress, anxiety, and psychological scores. Probiotic was also associated decreased cortisol, decreases in proinflammatory cytokines, and increases in anti-inflammatory cytokines. | 2019 | Chong HX, Yusoff NAA, Hor YY, et al. Benef Microbes. 2019;10(4):355-373. |
| Randomized, double-blind study investigated the effects of a multi-strain probiotic on brain functional and structural connectivity in 45 healthy subjects. Participants were randomized equally to probiotic, placebo, and control groups. Assessments: fMRI and diffusion MRI of the brain at baseline and again at 4 wk | Lactobacillus casei W56; L acidophilus W22; L paracasei W20; L salivarius W24; L plantarum W62; Lactococcus lactis W19; Bifidobacterium lactis W51;B lactis W52; B bifidum W23 | The probiotic group showed decreased functional activity in the MFGN and in the default mode network in the frontal lobe, and increased functional connectivity in the cingulate gyrus and precuneus cortex. These changes represented positive effects on behavior, attention, and decision making. | 2019 | Bagga D, Aigner CS, Reichert JL, et al. Eur J Nutr. 2019;58(5):1821-1827. |
Formulation Evidence
As evidenced by a wide body of research, including the human trials listed in Table 1, the mechanistic strengths and biological effects of probiotics are strain-specific. Thus, the performance of 1 strain cannot be extrapolated to another of the same genus and species. Moreover, evidence generated by a specific strain or strains is in part the result of the studied formulation itself in terms of CFU, complementary probiotic strain synergies, carrier matrix, gastric acid protection, and the presence of prebiotic support (synbiotics). Therefore, although both in-vitro and in-vivo evidence supporting individual strain efficacy for a specific indication is a useful screening tool, hypothesized performance can only be validated by human trials that use the final formulation. An informed formulation development process23 will screen for strain mechanisms that 1) most effectively address the physiological perturbations associated with a specific indication or set of symptoms; and 2) assess the hypothesized efficacy through both animal and human studies. Although not applicable to the majority of either consumer or professional probiotic brands, a number of commercial formulations have been studied in blinded, placebo-controlled fashion. Where available, manufacturers will openly share these data. Despite being somewhat more limited in value, in-vivo evidence in the form of case studies can also prove illuminating in terms of both performance and protocol.
Manufacturing Quality
US supplement manufacturers are regulated under the Dietary Supplement Health and Education Act (DSHEA) of 1994, which requires adherence to good manufacturing procedures (GMP). Adherence to the standards is easily identified by the manufacturers’ use of NSF International GMP certification mark. Beyond the GMP standard, there are several indications of quality manufacturing processes that support reliable efficacy. On request, your preferred probiotic manufacturer should be able to provide documented evidence to demonstrate the following:
- Strain safety and mechanistic strengths
- Absence of allergens/Presence of genetically modified ingredients
- Gastric survival and metabolic activity
- Viable probiotic count through expiration date
Summary
The gut-brain axis is a complex, bidirectional network that influences sympathetic and parasympathetic signaling between the GI tract and the brain. Intestinal microbiota, bacterial metabolites, and gram-negative endotoxin all have profound effects on gut inflammation, the stress response, brain neurotransmitters, and mood disorders such as depression and anxiety. Targeted probiotics/psychobiotics offer the promise of safe and effective mood and stress support. As naturopathic doctors, we have the responsibility to help guide our patients to the highest quality and most deeply studied formulations.
References:
- American Psychological Association. Stress in the Time of COVID-19. Volume One. May 2020. APA Web site. https://www.apa.org/news/press/releases/stress/2020/stress-in-america-covid.pdf. Accessed November 2, 2020.
- American Psychological Association. Stress in the Time of COVID-19. Volume Two. June 2020. APA Web site. https://www.apa.org/news/press/releases/stress/2020/stress-in-america-covid-june.pdf. Accessed November 2, 2020.
- American Psychological Association. Stress in the Time of COVID-19. Volume Three. July 2020. APA Web site. https://www.apa.org/news/press/releases/stress/2020/stress-in-america-covid-july.pdf. Accessed November 2, 2020.
- Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047-3052.
- Hsiao EY, McBride SW, Hsien, S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451-1463.
- Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124-136.
- Ogbonnaya ES, Clarke G, Shanahan F, et al. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psych. 2015;78(4):e7-e9.
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203-209.
- Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-148.
- Mertz HR. Overview of functional gastrointestinal disorders: dysfunction of the brain-gut axis. Gastroenterology Clin N Am. 2003;32(2):463-476.
- McVey Neufeld KA, Mao YK, Bienenstock J, et al. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 2013;25:183-188.
- Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698-3703.
- Lavdas AA, Blue ME, Lincoln J, Parnavelas JG. Serotonin promotes the differentiation of glutamate neurons in organotypic slice cultures of the developing cerebral cortex. J Neurosci. 1997;17(20):7872-7880.
- Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050-16055.
- Savignac HM, Kiely B, Dinan TG, Cryan JF. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil. 2014;26(11):1615-1627.
- Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132-1139.
- Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82(5):1516-1524.
- Bluthé RM, Walter V, Parnet P, et al. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317(6):499-503.
- Maes M, Twisk FN, Kubera M, et al. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J Affect Disord. 2012;136(3):909-917.
- Bailey MT, Dowd SE, Galley JD, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397-407.
- Ait-Belgnaoui A, Colom A, Braniste V, et al. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 2014;26(4):510-520.
- Sarkar A, Lehto SM, Harty S, et al. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39(11):763-781.
- Grumet L, Tromp Y, Stiegelbauer V. The Development of High-Quality Multispecies Probiotic Formulations: From Bench to Market. Nutrients. 2020;12(8):2453.

Jessica Brandes, ND received her doctorate of naturopathy from the National University of Natural Medicine (NUNM). She has worked extensively in primary care, and while living in London she worked in the area of population health, in partnership with the NHS. Dr Brandes maintains a small and specialized private practice in Portland, OR, and is actively engaged in advocacy work for SUDEP awareness.